Gepants for Migraine: An Update on Long-Term Outcomes and Safety Profiles
Article information
Abstract
Calcitonin gene-related peptide receptor antagonists, also referred to as gepants, represent a transformative advancement in migraine pharmacotherapy, providing both acute and preventive treatment options without the vasoconstrictive limitations of triptans. Since their initial approval in 2019, gepants have gained widespread clinical adoption, necessitating comprehensive evaluation of their long-term safety and efficacy. This review synthesizes current evidence on four calcitonin gene-related peptide receptor antagonists (rimegepant, atogepant, ubrogepant, and zavegepant) derived from pivotal trials, open-label extension studies, and real-world observational data. Rimegepant demonstrates sustained efficacy and minimal adverse events over 52 weeks, with no evidence of medication-overuse headaches or hepatotoxicity. Atogepant maintains progressive clinical benefits and favorable tolerability for up to 1 year, exhibiting low rates of treatment-emergent adverse events and discontinuation. Ubrogepant remains effective and well-tolerated during long-term intermittent use, with no clinically significant safety signals over extended exposure. Zavegepant, the first intranasal gepant, shows promising long-term tolerability, with the most frequently reported localized adverse event being transient dysgeusia. No consistent hepatic, cardiovascular, or serious systemic toxicity has emerged for any of the agents, and discontinuation rates due to adverse events remain consistently low. Current evidence supports gepants as safe and effective therapies for long-term migraine management, although ongoing surveillance and extended-duration studies remain essential to fully characterize their safety profile, particularly in high-risk populations and combination therapy scenarios. In conclusion, gepants offer a well-tolerated, non-vasoconstrictive alternative for migraine patients who require sustained treatment, representing a significant therapeutic advancement in migraine.
INTRODUCTION
Migraine is a common, disabling neurologic disorder driven in part by activation of the trigeminovascular system and release of neuropeptides such as calcitonin gene-related peptide (CGRP).1,2 CGRP is a critical mediator, which is released from trigeminal neurons during migraine attacks and potently dilates cranial blood vessels.3 Elevated CGRP levels have been correlated with migraine pain, and infusion of CGRP can trigger migraine in susceptible individuals.4 These insights led to the development of CGRP-targeted therapies, notably the CGRP receptor antagonists (gepants).
Gepants are small-molecule compounds (molecular weight <1 kDa) that selectively antagonize CGRP receptors, thereby preventing CGRP from binding and triggering the pro-migraine signaling cascade.5 These agents primarily inhibit CGRP signaling at peripheral sites outside the blood-brain barrier due to their minimal central nervous system penetration, effectively reducing neurogenic inflammation and pain transmission without inducing direct vasoconstriction.5 This mechanism represents a critical therapeutic advantage over triptans: while triptans cause significant vasoconstriction of cranial and coronary vessels, gepants achieve antimigraine efficacy without compromising vascular function.6 Although early first-generation gepants such as telcagepant demonstrated therapeutic promise, their development was discontinued due to hepatotoxicity concerns.7 The subsequent development of second-generation gepants has successfully addressed these safety issues, establishing a new paradigm in migraine-specific pharmacotherapy.7,8
Four CGRP receptor antagonists have received regulatory approval since 2019, heralding a transformative era in migraine therapeutics. Ubrogepant and rimegepant were the pioneering oral gepants approved for acute migraine management, with ubrogepant gaining approval in late 2019 followed by rimegepant in 2020.9-12 Both agents demonstrate rapid onset of pain relief while circumventing the cardiovascular contraindications that limit triptan use.13 Atogepant represents a distinct therapeutic advance as the first oral gepant specifically developed for migraine prophylaxis. Initially approved in 2021 for preventive treatment of episodic migraine with once-daily dosing, its indication was subsequently expanded in 2023 to include chronic migraine prevention.14,15 Zavegepant, classified as a third-generation gepant, introduced a novel delivery mechanism as the first intranasally administered CGRP antagonist, receiving approval in 2023 for acute migraine treatment.16 The nasal spray formulation of zavegepant offers distinct clinical advantages through rapid mucosal absorption and provides a valuable therapeutic option for patients experiencing nausea or vomiting during migraine episodes, circumstances that often preclude effective oral medication administration.16
With the expanding clinical adoption of gepants, a growing number of patients are receiving long-term therapy, generating considerable interest and concern regarding the efficacy, safety, and potential adverse events associated with prolonged use. This review focuses specifically on the safety profiles of gepants and their long-term clinical outcomes in migraine management. Our objective is to provide clinicians with a comprehensive, up-to-date analysis of long-term safety data and therapeutic outcomes for gepants, thereby facilitating informed decision-making when considering these agents for migraine patients.
LONG-TERM OUTCOMES WITH GEPANTS
1. Rimegepant
Rimegepant demonstrated sustained efficacy over extended treatment periods when administered every other day for migraine prevention and/or as-needed for acute migraine management.17 In a pivotal 52-week open-label study evaluating rimegepant for as-needed acute treatment, patients demonstrated a significant reduction in migraine frequency throughout the study period: monthly migraine days decreased from a baseline of 10.9 days to 8.9 days by week 52.17 During the open-label study, long-term preventive and acute rimegepant treatment consistently reduced migraine frequency throughout the 52-week period, with patients experiencing a decrease from a baseline mean of 9.9 monthly migraine days to an average reduction of 6.2 days per month.18 The proportion of patients achieving ≥50% reduction in mean moderate or severe monthly migraine days progressively increased from 63.6% during weeks 1–4 to 80.9% during weeks 49–52. Comparable improvements were observed for ≥75% reductions (increasing from 44.1% to 65.8%) and complete elimination of moderate to severe migraine days (increasing from 25.6% to 49.3%).19 Throughout the 52-week treatment period, preventive and/or acute rimegepant therapy yielded significant improvements in quality of life, as evidenced by enhanced scores across improved EuroQol-5 Dimensions-3 Level utility values and multiple domains of the Migraine-Specific Quality of Life (MSQoL).20 There was no evidence of medication-overuse headache development and migraine frequency remained stable or decreased despite some patients utilizing rimegepant on a near-daily basis.21,22 In the long-term open-label extension study, rimegepant 75 mg taken as-needed up to once daily for acute migraine treatment showed that mean monthly tablet utilization remained stable or trended downward over 1 year of follow-up, decreasing from 7.9 tablets in weeks 4–8 to 7.3 tablets in weeks 48–52.17 This contrasts markedly with the long-term use of triptans or analgesics in comparable populations, which frequently results in escalating headache frequency and medication-overuse headache development. The distinctive dual acute-preventive properties of rimegepant appear to be preserved with sustained use, representing a significant therapeutic advantage in migraine management. In contrast, Croop et al.21 primarily focused on long-term safety assessments. The efficacy outcomes were restricted to patient-reported measures, including MSQoL, medication preference, patient satisfaction, and clinical global improvement relative to baseline, and did not provide detailed reporting on reductions in headache attack frequency.21
2. Atogepant
Atogepant is developed exclusively as a preventive treatment option for migraine.23 The 52-week open-label trial of once-daily atogepant 60 mg demonstrated progressive and sustained efficacy in migraine prevention, with mean monthly migraine days reduction increasing from –3.8 during weeks 1–4 to –5.2 at weeks 49–52.24 The proportion of participants achieving clinically meaningful response rates showed marked improvement over time: ≥50% monthly migraine days reduction increased from 60.4% early in treatment to 84.2% by study end, while ≥75% and 100% reduction rates similarly improved from 37.2% and 20.7% to 69.9% and 48.4%, respectively.24 These findings demonstrate that atogepant provides not only immediate preventive benefits but also enhanced efficacy with continued long-term use, establishing its durability as a migraine-specific preventive therapy. Another positive outcome is sustained improvements in patient-reported outcomes, with MSQoL scores showing least-squares mean changes from baseline of 30.0 (95% confidence interval [95% CI], 28.2–31.9) at week 12, further improving to 34.7 (95% CI, 32.7–36.7) at week 52.25 Significant improvements were also observed across other MSQoL domains, as well as in Activity Impairment, Productivity Impairment, and Headache Impact Test-6 total scores throughout the study period. These findings demonstrate that atogepant provides progressive and durable benefits in migraine-related quality of life and functional outcomes, with improvements maintained and enhanced over long-term treatment.25 The sustained patient-reported outcome improvements complement the clinical efficacy data, supporting atogepant’s role as an effective preventive therapy that meaningfully impacts patients’ daily functioning and well-being.24,25
3. Ubrogepant
The 52-week extension study following the 12-week pivotal studies (ACHIEVE I and II trials)26,27 demonstrated sustained therapeutic efficacy, with 2-hour pain freedom achieved in approximately 23% (50 mg) and 25% (100 mg) of treated attacks, while 2-hour pain relief was observed in 65%–68% of cases.28,29 Efficacy was markedly superior when treating mild-intensity attacks during a 52-week treatment period. In a within-subject analysis, ubrogepant 50 mg and 100 mg demonstrated significantly higher 2-hour pain freedom rates when taken at the mild headache (50 mg: 51.2%, 100 mg: 54.3%) compared to the moderate/severe headache (50 mg: 24.6%, 100 mg: 27.2%).30 These findings suggest that early treatment with ubrogepant leads to improved efficacy in acute migraine management.30
LONG-TERM SAFETY PROFILES WITH GEPANTS
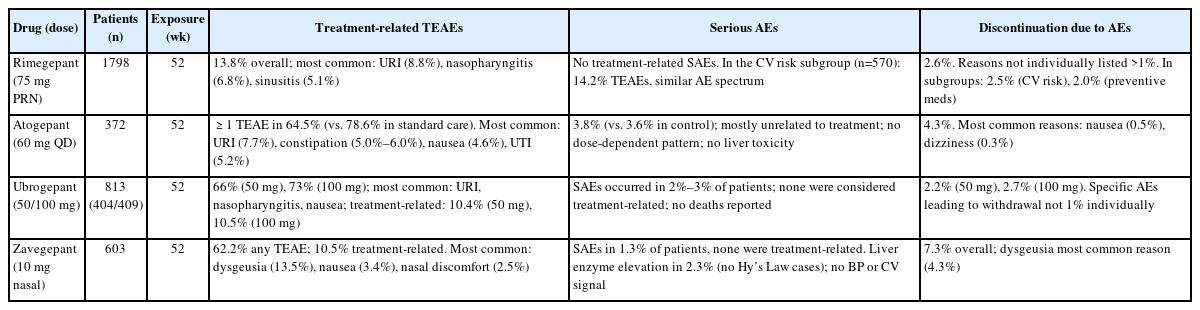
An overview of long-term safety data from open-label studies of rimegepant, atogepant, ubrogepant, and zavegepant is summarized in Table 1.
1. Rimegepant
Long-term safety profile has been characterized through a large open-label extension study and supported by real-world registry data and subgroup analyses.21,22,31,32 A 52-week, open-label study evaluated the long-term safety of rimegepant 75 mg as-needed therapy in 1,798 adults with migraine.21 The results demonstrated good tolerability, with 13.8% of participants experiencing treatment-emergent adverse events (TEAEs) considered drug-related. The most frequently reported TEAEs were mild and included upper respiratory tract infection (8.8%), nasopharyngitis (6.8%), and sinusitis (5.1%). Treatment discontinuation due to adverse events occurred in 2.7% of patients, and serious adverse events (SAEs) were reported in 2.6% of patients, with drug-related SAEs in 0.6%. Importantly, no cases of drug-induced liver injury were identified, confirming rimegepant’s hepatic safety profile during long-term intermittent use. In addition to the primary open-label study,21 two subgroup analyses have been reported.31,32 True et al.31 analyzed 570 patients stratified by cardiovascular risk factors, showing that the long-term safety and tolerability of rimegepant were consistent regardless of baseline cardiovascular risk, with treatment-related TEAEs occurring in 14.2% of patients and discontinuation due to adverse events in 2.5%. Similarly, Berman et al.32 evaluated 695 patients stratified by concomitant preventive medication use. The rate of treatment-related TEAEs classified as related to rimegepant was comparable between the cohort using preventives (22.2%) and the cohort not using them (19.7%). The incidence of serious treatment-related AEs was also low in both groups, occurring in 1.6% and 0.4% of participants, respectively.32 These subgroup findings support that the favorable safety profile of rimegepant is preserved across clinically relevant patient groups. Real-world validation comes from the GAINER study, a prospective, multicenter Italian study evaluating rimegepant for acute migraine treatment.22 This study demonstrated adverse events reported in 15.5% of participants and no SAEs documented. Patient-reported tolerability was rated as good or excellent in 85.4% of patients, and no treatment discontinuations due to adverse events were reported.22
2. Atogepant
Safety of atogepant was established in 12-week phase 3 studies and further supported by open-label extension studies. There were open-label 52-week and 40-week long-term safety studies with an ongoing follow-up to 156 weeks (NCT04686136). Participants who completed one of pivotal studies were eligible to participate in the long-term safety studies.24,33 In a 52-week randomized open-label trial of once-daily atogepant 60 mg (n=744), ≥1 TEAE was reported in 67.0% of participants, most commonly upper respiratory tract infection (10.3%), constipation (7.2%), nausea (6.3%), and urinary tract infection (5.2%). Serious TEAEs occurred in 4.4% and discontinuations due to adverse events in 5.7%.24
In a separate 40-week open-label extension of the ADVANCE trial (n=685), ≥1 TEAE occurred in 62.5% of participants.33 The most common TEAEs were upper respiratory tract infection (5.5%), urinary tract infection (5.3%), nasopharyngitis (4.8%), sinusitis (3.6%), constipation (3.4%), and nausea (3.4%). Serious TEAEs occurred in 3.4% of patients, none considered treatment-related, and discontinuation due to adverse events occurred in 3.2%. In this Ashina et al.’s study,24 efficacy outcomes were not collected, as this extension focused solely on safety.
From open-label extension studies, atogepant is well tolerated, with few patients requiring treatment cessation due to adverse events, and that most side effects are manageable within clinical practice. Atogepant 60 mg once daily has been reported to induce clinically meaningful weight loss in patients with migraine who are overweight or obese, as presented at the 2025 American Headache Society Annual Scientific Meeting and supported by data from the ongoing long-term extension study (NCT04686136). Approximately one-third of participants achieved a ≥5% reduction in body weight after 52 weeks, with a mean weight loss of 3.4 kg.
3. Ubrogepant
A 52-week phase 3 extension study provides robust evidence for ubrogepant’s long-term safety profile in acute migraine treatment.28 The study enrolled 813 participants (404 receiving 50 mg, 409 receiving 100 mg) who collectively treated 21,454 migraine attacks with 31,968 doses of ubrogepant.28 Overall TEAEs were reported in 66% of patients receiving 50 mg and 73% receiving 100 mg. However, the majority of these events were mild to moderate in severity, with the most frequently reported being upper respiratory tract infection, nasopharyngitis, and nausea. Treatment-related TEAEs remained low across both dosing groups, occurring in 10.4% of patients receiving 50 mg and 10.5% receiving 100 mg. SAEs occurred in 2%–3% of patients, and treatment discontinuation due to adverse events was low (2.2% in the 50 mg group and 2.7% in the 100 mg group). No deaths occurred during the trial period. Real-world safety data from the VigiAccess and U.S. Food and Drug Administration’s adverse event reporting system databases provide valuable post-marketing insights.34 Through March 2024, 3,478 adverse event reports associated with ubrogepant were identified. The most frequently reported adverse events in post-marketing surveillance included nausea (4.7%), fatigue (1.8%), vomiting (1.6%), and headache (0.8%). Hepatobiliary adverse events were rare, with no strong positive safety signal detected. Cardiovascular events and severe hypersensitivity reactions were infrequent, corroborating the safety profile observed in controlled clinical trials.34
4. Zavegepant
Given the recent approval of zavegepant, real-world data are currently scarce, and long-term safety assessments rely largely on findings from one open-label study.35 The long-term safety of zavegepant nasal spray 10 mg for the acute treatment of migraine was evaluated in a 52-week, open-label, phase 2/3 study involving 603 adult participants. Over the study period, 21,052 migraine attacks were treated with 48,504 doses of zavegepant.35 TEAEs were reported in 76.1% of patients, most commonly dysgeusia (39.1%), nasal discomfort (10.3%), COVID-19 infection (7.5%), nausea (6.1%), nasal congestion (5.5%), throat irritation (5.5%), and back pain (5.3%). Discontinuation due to adverse events occurred in 6.8% of participants, most frequently from dysgeusia (1.5%). Severe adverse events were reported in 3.6% and SAEs in 1.2%, none considered treatment-related. Alanine transaminase or aspartate transaminase elevations >3× upper limit of normal were observed in 2.6% of patients, but no Hy’s law cases occurred. Importantly, no cases of medication-overuse headache, cardiovascular events, or suicidality-related adverse events were identified, supporting the favorable long-term safety profile of zavegepant.35
SPECIAL ISSUES
1. Combination treatment with calcitonin gene-related peptide monoclonal antibodies
Recent evidence supports the feasibility and safety of combining gepants with CGRP monoclonal antibodies (mAbs). The COURAGE real-world observational study showed that ubrogepant, when used in patients already receiving an anti-CGRP mAb, provided meaningful pain relief and return to normal function, with high levels of patient satisfaction and treatment optimization, and without new safety concerns.36 In line with these findings, a retrospective analysis of 234 patients treated with rimegepant or ubrogepant in addition to CGRP mAbs reported that the combination was generally well tolerated, with only mild and transient adverse events that did not necessitate discontinuation.37 These studies suggest that combination therapy targeting the CGRP pathway at different sites may be a safe and practical treatment strategy, although larger prospective randomized trials are still needed to confirm long-term safety and efficacy.
2. Efficacy in patients with prior failure of acute migraine therapies
Emerging data indicate that gepants remain effective for acute migraine relief in patients who have previously experienced insufficient response or tolerability with triptans. A pooled post hoc analysis of three phase 3 trials found that rimegepant 75 mg provided comparable rates of pain freedom and most bothersome symptom relief at 2 hours in participants with inadequate response to one or more triptans, current triptan users, and triptan-naive individuals (p≤0.013).38 Moreover, long-term safety and preference for rimegepant were consistent across subgroups with a history of triptan discontinuation.39 These findings underscore the clinical value of gepants as a well-tolerated and effective alternative for migraine patients with prior acute treatment failures. However, focused prospective studies in these refractory subpopulations remain warranted.
3. Effectiveness in traditionally suboptimal responders: medication-overuse headache and psychiatric comorbidity
Gepants appear to be particularly advantageous in traditionally challenging migraine subgroups, such as patients at risk of medication-overuse headache and those with psychiatric comorbidities. Crucially, long-term use of rimegepant (up to 52 weeks as needed [pro re nata]) has been associated with a sustained reduction in monthly migraine days without increases in monthly medication usage, suggesting a low risk of medication-overuse headache development.17 Regarding psychiatric comorbidities, adults with migraine and histories of anxiety and/or depression tolerated rimegepant well, demonstrating favorable safety and tolerability profiles.40 These findings support gepants as effective and well-tolerated options for migraine management in populations traditionally considered suboptimal responders, although further targeted prospective studies are warranted.
CURRENT EVIDENCE GAPS AND FUTURE DIRECTIONS
While gepants have established themselves as both effective and safe for long-term migraine management, several critical questions remain unresolved. First, comprehensive safety data extending beyond 1–2 years remain limited. The available safety evidence through 1 year of treatment is reassuring, demonstrating no significant organ toxicity or increased incidence of adverse events. However, migraine frequently represents a lifelong condition requiring decades of preventive intervention, and the consequences of sustained CGRP receptor blockade over such extended periods remain incompletely understood. Given CGRP’s widespread expression across multiple organ systems including cardiovascular, gastrointestinal, and endocrine tissues prolonged receptor inhibition may reveal subtle physiological effects that are not apparent in shorter-term studies, necessitating continued long-term surveillance and research.41 Future studies, including a 3-year atogepant safety trial (NCT04686136) will be essential for detecting any late-emerging adverse events.
Second, the cardiovascular safety profile of gepants in high-risk populations represents one of the most significant unresolved questions in migraine therapeutics. Most pivotal clinical trials systematically excluded patients with significant cardiovascular disease, creating a substantial evidence gap regarding gepant safety in individuals with active coronary artery disease, cerebrovascular conditions, or multiple vascular risk factors. This exclusion of high-risk cardiovascular patients from foundational studies means that formal safety evaluation in real-world populations with established vascular disease remains incomplete. Critical questions persist regarding long-term cardiovascular outcomes during gepant therapy, including potential effects on blood pressure regulation, risk of vascular events, and safety in patients with compromised cardiovascular reserve. Limited observational data suggest that gepants may be well-tolerated in patients with cardiovascular risk factors.31 Long-term registries tracking vascular outcomes, blood pressure changes, and cardiac events during gepant therapy are essential to address this knowledge gap. In addition, it should be noted that most available long-term safety data are derived from open-label extension studies and industry-sponsored clinical trials, which may introduce a higher risk of bias compared with randomized controlled trials.
CONCLUSION
Gepants represent a significant therapeutic advancement in migraine management, offering robust efficacy for both acute and preventive treatment with an excellent safety profile. Clinical trials and real-world evidence consistently demonstrate that rimegepant, ubrogepant, atogepant, and zavegepant are well-tolerated across diverse patient populations, avoiding the cardiovascular contraindications associated with traditional therapies. The available safety data support sustained therapeutic benefit with minimal long-term concerns, enabling patients to achieve improved quality of life even with extended use. As clinical experience continues to expand, ongoing pharmacovigilance remains essential to monitor for rare or delayed adverse events and ensure the continued safety of this promising therapeutic class.
Notes
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: SC; Data curation: SC; Investigation: SC, KC; Writing–original draft: SC, KC; Writing–review & editing: SC, KC.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.