Interictal Burden of Migraine: A Narrative Review
Article information
Abstract
Migraine is a chronic neurological disorder associated with substantial disability and societal costs. Traditionally, research and clinical care have focused on the ictal phase, characterized by headache and accompanying symptoms. However, growing evidence suggests that a considerable portion of migraine-related disability occurs between attacks, known as the interictal burden (IIB). IIB encompasses a wide spectrum of cognitive, emotional, sensory, and functional impairments that persist during headache-free periods, including fatigue, allodynia, photophobia, cognitive dysfunction, anticipatory anxiety, and social withdrawal. These symptoms can markedly reduce quality of life, work productivity, and family functioning, even in individuals with infrequent attacks. In a descriptive survey of 506 migraine respondents, 67% experienced severe IIB. The effects of IIB extend beyond patients themselves, contributing to presenteeism in the workplace and imposing emotional and logistical strain within families. Several instruments, including the Migraine Interictal Burden Scale (MIBS-4), Migraine-Specific Quality of Life Questionnaire (MSQ v2.1), Headache Impact Test (HIT-6), and Migraine Disability Assessment (MIDAS), have been employed to assess different dimensions of IIB. Nonetheless, no single comprehensive and standardized tool fully captures the multidimensional nature of IIB. Recognizing and addressing IIB is essential for delivering holistic, patient-centered migraine care. Future research should focus on developing validated assessment instruments and incorporating IIB measures into clinical trials and routine practice to better understand and alleviate the hidden burden of migraine.
INTRODUCTION
Migraine is a disabling chronic neurological disease characterized by episodic attacks consisting of headache and non-pain symptoms such as nausea, sensory hypersensitivities, mood changes, and cognitive dysfunction.1,2 Previous studies have demonstrated that migraine imposes a substantial burden on patients, families, the workplace, and society.3,4 At the population level, “migraine-attributed burden” is defined as the sum of the negative impact of migraine on individuals with migraine plus the impact on people without migraine.5 Migraine most commonly occurs between the second and sixth decades of life, which are crucial years for education, career development, and productivity.6,7 Understanding the magnitude of and contributors to both ictal and interictal migraine burden is essential for accurately assessing the true impact of migraine and developing targeted interventions to reduce it.8,9 While its burden has traditionally been quantified based on ictal features such as attack frequency, severity, and duration, a growing body of research emphasizes that migraine is not confined to the ictal phase. Interictal burden (IIB) refers to the constellation of symptoms and restrictions experienced between attacks, including sensory hypersensitivity, cognitive impairment, anticipatory anxiety, and impaired social and occupational functioning.10 For this review, the interictal period is defined as a headache-free interval of at least 24 hours since the last migraine ictus, excluding prodromal and postdromal phases. Prior studies have adopted varying definitions. For example, Lampl et al.11 described IIB as the “loss of health or wellbeing attributable to a headache disorder reportedly experienced while headache-free, affecting all areas of life on any day." Peng and May,12 from a clinical perspective, characterized the interictal phase as the interval between two attacks during which patients are “usually relatively symptom free”. Such heterogeneity in definitions may influence estimates of IIB, particularly in chronic migraine (CM) where headache-free days are scarce.
The objective of this narrative review is to provide an overview of IIB in migraine, focusing on its epidemiology, domains of impact, assessment tools, clinical implications, and future directions.
METHODS
This article was prepared as a narrative review to provide an overview of current knowledge on IIB in migraine. The review was structured and reported according to the SANRA (Scale for the Assessment of Narrative Review Articles) guidelines to ensure clarity, transparency, and scientific rigor.
We performed a literature search using MEDLINE (via PubMed) and Embase databases. The search covered the period from January 1, 2000 to September 1, 2025, with no geographic restrictions. The search strategy combined controlled vocabulary (MeSH/Emtree terms) and free-text keywords related to migraine and IIB. The core search string included terms such as: “migraine” OR “headache disorders” AND (“interictal burden” OR “interictal symptoms” OR “migraine burden” OR “anxiety” OR “cognitive impairment” OR “presenteeism” OR “quality of life”).
Relevant literature was identified through the review of key published studies and large epidemiological projects as well as other peer-reviewed research articles and systematic reviews. Articles were selected for their relevance to the main themes of this review: (1) epidemiology of IIB, (2) domains of impact (cognitive, psychological, sensory, social, and functional), (3) assessment tools used to measure IIB, and (4) clinical and societal implications.
Inclusion criteria were peer-reviewed English-language publications that reported data on people with migraine, including both episodic and CM, and addressed at least one of the themes above. Exclusion criteria included abstracts without full text, case reports with fewer than 10 participants, and studies unrelated to migraine or IIB.
As this was a narrative review, a formal risk-of-bias tool was not applied. However, study design and methodological quality were considered when interpreting findings, and greater weight was given to systematic reviews and large observational studies.
1. Epidemiology of interictal burden
Epidemiological surveys demonstrate that IIB is common and clinically meaningful. The OVERCOME (Japan) study reported that approximately 41.5% of respondents with migraine had moderate-to-severe IIB, and a comparable proportion (53.8%) was seen in OVERCOME (US).13 Similarly, European data from the Eurolight study revealed that 26.0% of individuals with migraine reported IIB, including 10.6% with interictal anxiety and 14.8% with avoidance behaviors.11 CM is consistently associated with higher interictal impairment than episodic migraine.
2. Domains of interictal burden
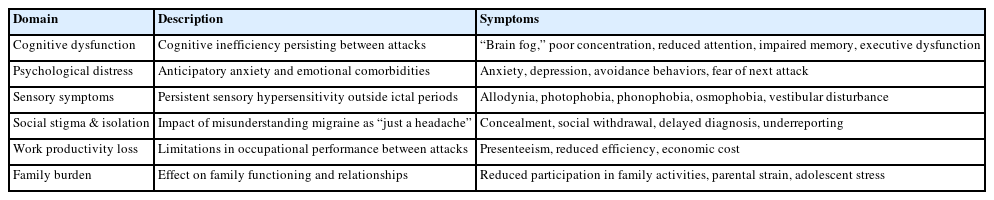
IIB can be categorized into several key domains, each contributing to a patient’s overall disability and reduced quality of life (QoL) (Table 1).
1) Cognitive and behavioral dysfunction
Many patients report cognitive impairments during the interictal phase, often described as “brain fog.” These difficulties may include reduced selective attention and deficits in executive function, impacting the ability to concentrate, remember, and perform complex tasks.14 One study found evidence of mild executive dysfunction in patients with migraine without aura during the interictal period.15 Patients with CM demonstrate poorer frontal lobe–related cognitive performance, especially in executive function, compared with episodic migraine and healthy controls. Deficits in executive tasks such as the Trail Making Test and Wisconsin Card Sorting Test have been documented in CM, and higher migraine severity has been associated with reduced attention and slower processing speed.15-18
2) Psychological distress
The constant threat of an impending attack often leads to anticipatory anxiety, which may result in avoidance behaviors that limit daily activities and affect the ability to make plans or commitments. Anxiety and depression may be associated with more severe migraine disease. In the Nord-Trøndelag Health Study (HUNT) cohort study, anxiety was associated with a two-fold increased risk and depression with a 2.6-fold increased risk of developing medication overuse headache (MOH).19 Depression is associated with a higher risk of transformation from episodic to CM, the risk of which is greatest among those with more severe depression.20
3) Social stigma and isolation
Migraine is frequently misunderstood as a simple headache, leading to social stigma. Patients may hide their condition from colleagues, friends, and family, resulting in social isolation and feelings of being misunderstood.21 Stigma also contributes to underreporting and delays in diagnosis, while reinforcing avoidance behaviors. In the OVERCOME (US) cohort, 45.1% of migraine patients reported ever hesitating to seek care, with hiding migraine and perceived stigma being among the strongest associated factors.22 Higher stigma scores were strongly associated with greater disability, poorer QoL, and reduced care seeking.23 Over time, these dynamics can affect career advancement, education, and family planning.24 Additionally, stigma may discourage patients from seeking treatment, exacerbating the emotional burden and resulting in unnecessary suffering from untreated migraine.24,25 To better quantify stigma, the migraine-related stigma (MiRS) questionnaire was recently developed and validated using OVERCOME data, providing a standardized tool for measuring perceived stigma and its clinical impact.26
4) Persistent non-pain symptoms
Many symptoms associated with the ictal phase—such as allodynia, photophobia, phonophobia, osmophobia, vestibular disturbances and motion sickness—can persist into the interictal phase at lower intensity. In the OVERCOME (US) cohort, 31.7% of participants reported frequent interictal symptoms such as sensitivity to light or sound, cognitive difficulties, and fatigue, even on non-headache days.8,23 Neuroimaging studies, including functional magnetic resonance imaging and structural analyses, demonstrate persistent cortical hyperexcitability and alterations in regional brain structure in pain-processing regions and regions responsible for processing other sensory stimuli, supporting a neurobiological basis for these ongoing symptoms.9,27,28
5) Work and productivity loss
Workplace productivity is profoundly affected by migraine, especially through presenteeism—working while symptomatic but at reduced capacity. One study estimated that 89% of total productivity loss due to migraine was attributable to presenteeism rather than absenteeism.29 In the Japanese OVERCOME cohort, presenteeism accounted for a substantial portion of work time impairment—up to 49.9% of work hours—while absenteeism rates remained low (3.8%–6.2%). This suggests that presenteeism-related costs far exceed those from absence in this population. Although direct data linking interictal fatigue or reduced vitality to presenteeism costs in Japanese cohorts are lacking, the high proportion of productivity impairment attributable to presenteeism supports the possibility that interictal symptoms contribute meaningfully to economic burden.30,31 Furthermore, higher Migraine Interictal Burden Scale (MIBS-4) scores have been shown to correlate with greater activity impairment and lower workplace productivity.8,32
6) Family and social burden
Longitudinal data from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study highlight the widespread impact of migraine on family functioning. Nearly half of respondents with migraine reported reduced participation in family activities, with the highest burden observed among those with CM. They felt their partner did not understand the severity of their condition, and a substantial proportion believed they would be better parents without migraine.33 Furthermore, adolescents living with a parent with CM experienced greater anxiety, missed activities, and assumed more household responsibilities than those with a parent who had episodic migraine.34 In a study of headache specialty clinic patients with migraine in the United States, 19.9% of women avoided pregnancy due to their migraine, mostly because of concerns about negative impacts of migraine on their pregnancy and child.35 These findings demonstrate that migraine is not only a personal condition but also a family disease, with implications for emotional health and household dynamics.
3. Assessment tools for interictal burden
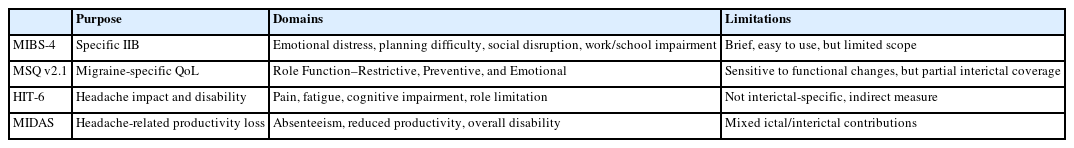
Recognition of IIB as a major contributor to migraine-related disability has led to the development and adaptation of various assessment tools. Although a fully comprehensive, validated instrument for IIB remains unavailable, both migraine-specific and general instruments are used (Table 2).
1) Migraine-specific instruments
Most migraine-specific instruments were originally developed to assess global headache-related disability, with particular emphasis on ictal burden. While not specifically designed to evaluate interictal effects, certain items—such as those addressing fatigue, concentration difficulties, or reduced vitality—may inadvertently capture functional limitations that persist during headache-free intervals. Accordingly, these measures should be interpreted as providing only partial and indirect insights into IIB rather than as dedicated assessments. This limitation highlights the need for rigorously validated instruments explicitly designed to quantify IIB, such as the MIBS-4.
(1) Migraine Interictal Burden Scale
The MIBS-4 is currently the only instrument explicitly designed to assess IIB. It comprises four items assessing emotional distress, difficulty in making plans or commitments, social or leisure disruption, and impairment in work or school life. Each item is rated on a 0–3 scale (total score 0–12), with higher scores indicating greater burden. Scores are categorized as none (0), mild (1–2), moderate (3–4), or severe (≥5). Validation data from real-world studies such as OVERCOME (Japan, US) show correlation with health-related QoL, productivity loss, and daily functioning.9 In the OVERCOME (Japan) study, higher MIBS-4 scores were associated with greater activity impairment, productivity loss, absenteeism, and presenteeism within Headache Impact Test (HIT-6) strata.8
(2) Migraine-Specific Quality of Life Questionnaire
The Migraine-Specific Quality of Life Questionnaire (MSQ v2.1) is a widely used instrument for assessing the impact of migraine on health-related QoL. It consists of three domains: Role Function–Restrictive, Role Function–Preventive, and Emotional Function. While originally developed to measure ictal-related quality-of-life impairment, items within the Role Function–Restrictive domain are sensitive to interictal functional limitations, such as reduced energy, motivation, and social participation on non-headache days.36 In a validation study of the Greek version of MSQ v2.1, significant moderate correlations were observed between MSQ scores and the Migraine Disability Assessment (MIDAS), with correlation coefficients ranging from ρ=–0.562 to –0.519 (p<0.001).37 These findings support that the Role Function–Restrictive domain reflects functional limitations in daily life and is relevant for assessing the broader impact of migraine beyond headache episodes.36
(3) Headache Impact Test
HIT-6 measures headache-related disability, including difficulty concentrating, fatigue, and role limitations. Although not designed specifically for IIB, psychometric studies suggest that some items indirectly reflect interictal cognitive and functional impairment.38,39
(4) Migraine Disability Assessment
MIDAS assesses productivity loss over the past three months due to migraine. Although it aggregates both ictal and interictal days, it can indirectly reflect persistent functional limitations in individuals with frequent attacks, providing a partial estimate of IIB.40,41
2) Generic and complementary instruments
In addition to migraine-specific tools, several broadly applicable instruments can be utilized to capture specific domains of IIB that are otherwise underrepresented (Table 3).
(1) Cognitive Failures Questionnaire
The Cognitive Failures Questionnaire (CFQ) is a self-reported survey that assesses daily memory, attention, and execution failures. In migraine patients, CFQ scores have been shown to correlate significantly with subjective cognitive impairment during attacks, supporting its utility as a complementary tool for evaluating cognitive dysfunction.42
(2) Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) assesses anxiety (HADS-A) and depression (HADS-D), which are key components of anticipatory anxiety and emotional distress between migraine attacks. In a cross-sectional study, individuals with migraine scoring ≥11 on HADS-A had over twice the odds of interictal anxiety compared to those with lower scores. Elevated HADS-A and HADS-D scores are also common in patients with MOH and significantly decrease following detoxification treatment, highlighting their clinical relevance. Furthermore, higher monthly headache days are associated with a dose-dependent increase in psychological distress, with ≥3 days linked to anxiety and ≥19 days to depression and severe disability.43-45
(3) Work Productivity and Activity Impairment questionnaire
The Work Productivity and Activity Impairment questionnaire (WPAI) measures absenteeism, presenteeism, and overall activity impairment.46 In Japan, presenteeism has been shown to consume 29.8%–49.9% of work time in migraine patients, far exceeding absenteeism rates (3.8%–6.2%).31 WPAI scores have also been found to increase with headache frequency, with presenteeism rising from 41.7% in patients with 0–3 monthly headache days to 67.5% in those with ≥15 days.47
(4) World Health Organization Disability Assessment Schedule 2.0 and 36-item short-form/EuroQol-5 Dimensions
Generic health-related QoL instruments such as World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0), 36-item short-form (SF-36), and EuroQol-5 Dimensions (EQ-5D) have been used in headache and pain research to capture interictal functional impairments, particularly in domains of vitality, social participation, and role limitations.48-50 A large European study demonstrated that higher frequency of headache days (≥4 monthly headache days) is associated with significantly lower health-related QoL (SF-6D, EQ-5D, and SF-36 summary scores) and increased work/activity impairment.51 Clinical data using 5-level EQ-5D (EQ-5D-5L) confirm that patients with CM report lower health utility scores compared to those with episodic migraine, even outside of headache episodes.39 Population-based data from England using SF-36 show broad impairment across vitality, role limitation, and social functioning domains among those with migraine, particularly in those with moderate to severe disability.52
DISCUSSION
The construct of IIB in migraine demonstrates that the impact from migraine extends beyond the ictal phase, encompassing persistent cognitive, emotional, sensory, and functional impairments even during headache-free periods. Epidemiological studies have shown that IIB is highly prevalent across all migraine subtypes, even including individuals with low-frequency episodic migraine. This highlights the limitation of traditional metrics, such as monthly headache days, which primarily focus on ictal symptoms and do not fully capture the full burden of migraine. Even when headache frequency is relatively low, the psychological burden of anticipating the next attack, along with ongoing symptoms such as fatigue, allodynia, photophobia, and cognitive dysfunction, can significantly reduce health-related QoL. These findings highlight the need to redefine migraine as a persistent neurological disorder rather than an episodic condition. They also emphasize the importance of integrating IIB into clinical assessment and treatment strategies.
IIB has widespread functional and societal implications. Beyond the direct suffering experienced by those with migraine, migraine significantly impacts the workplace, family life, and social interactions. Reduced workplace productivity is a major concern, with presenteeism consistently identified as the primary driver of economic loss. IIB not only affects individuals, but also imposes a substantial economic burden on employers and society.
The impact of migraine on families is also profound. The CaMEO study demonstrates that migraine interferes with family activities and relational dynamics, with the highest burden observed among those with CM. These findings emphasize that migraine is a family disease, influencing relationships, emotional well-being, and the daily functioning of household members.
Assessing IIB remains challenging due to methodological limitations. The MIBS-4 is the only tool specifically designed to measure IIB. Other instruments such as MSQ v2.1, HIT-6, and MIDAS were originally developed for ictal assessment but can provide partial insights into interictal effects. In addition, generic tools like the CFQ, HADS, WPAI, WHODAS 2.0, SF-36, and EQ-5D can complement migraine-specific measures by evaluating cognitive, psychological, and social dimensions. However, there is no single comprehensive tool that addresses all aspects of IIB, and many instruments lack cross-cultural validation, particularly for patients with CM. Furthermore, IIB is rarely included as a primary outcome measure in clinical trials, limiting our understanding of its responsiveness to treatment. Nonetheless, recent evidence indicates that IIB can be improved by preventive therapies. In a prospective cohort of 150 CM patients, onabotulinumtoxinA treatment reduced MIBS-4 scores by approximately 29% at 3 months and 42% at 12 months, reflecting sustained improvements in daily functioning.53 Similarly, clinical data with CGRP monoclonal antibodies, such as galcanezumab, demonstrate reductions in interictal symptoms including allodynia and fatigue.54 These findings highlight the potential of recent preventive treatments to alleviate both ictal and IIB, underscoring the need to systematically include IIB endpoints in future trials and clinical practice to provide a more comprehensive and patient-centered assessment of therapeutic benefit.
To address these gaps, several key steps are needed. First, a standardized definition of the interictal period should be established, particularly for CM, where headache-free days are less common. Second, new multidimensional instruments must be developed to comprehensively assess the cognitive, emotional, sensory, and social aspects of IIB and validated across diverse cultures and age groups. Third, both clinical trials and real-world registries should incorporate IIB measures to better evaluate its prognostic value and the effectiveness of preventive and behavioral interventions on reducing IIB. Finally, future research should investigate how cultural and sociodemographic factors influence the perception and reporting of IIB, thereby enhancing the global relevance and applicability of migraine management strategies.
In conclusion, IIB is a substantial yet underrecognized component of migraine-related burden. Even in the absence of headache, individuals with migraine may experience persistent symptoms that significantly impact QoL, work productivity, and family relationships. The development of comprehensive, validated assessment tools and the integration of IIB into longitudinal studies and clinical trials are essential steps toward addressing this hidden burden.
Notes
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: SKK, TJS; Data curation: SKK; Formal analysis: SKK; Investigation: SKK; Methodology: SKK; Supervision: TJS; Writing–original draft: SKK; Writing–review & editing: SKK, TJS.
CONFLICT OF INTEREST
Soo-Kyoung Kim is the Deputy Editor of Headache and Pain Research and was not involved in the review process of this article.
Todd J. Schwedt has received personal compensation for consulting with AbbVie, Linpharma, Lundbeck, and Salvia, and royalties from UpToDate, within the prior 24 months. He holds stock options in Allevalux and Nocira. His institution has received research grants on his behalf from American Heart Association, Flinn Foundation, Henry Jackson Foundation, National Headache Foundation, National Institutes of Health, Patient Centered Outcomes Research Institute, Pfizer, Spark Neuro, and the United States Department of Defense.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.