Genetic Architecture of Migraine: From Broad Insights to East Asian Perspectives
Article information
Abstract
Migraine is a complex neurological disorder with a strong genetic component, ranging from rare monogenic forms, such as familial hemiplegic migraine (FHM), to common polygenic migraine. FHM is primarily caused by mutations in CACNA1A, ATP1A2, and SCN1A, which affect ion channel function and cortical excitability. Additional genes, including PRRT2, have also been implicated, broadening the genetic landscape of monogenic migraine. Genome-wide association studies (GWAS) have identified multiple susceptibility loci for common migraine, highlighting key pathways related to neuronal excitability and vascular function. These findings have reinforced the neurovascular hypothesis of migraine pathogenesis. GWAS on other headache disorders, such as broadly defined headache or cluster headache, have also revealed both overlapping and distinct genetic risk factors. Genetic studies in East Asians have identified both ancestry-specific risk variants and overlapping loci with European populations, suggesting similarities in biological pathways while also highlighting population-specific differences in migraine susceptibility. Expanding research on the genetics of migraine in East Asian populations is essential for uncovering novel risk factors and improving the generalizability of genetic findings.
INTRODUCTION
Migraine is a prevalent and debilitating neurological disorder diagnosed based on clinical features of headache episodes.1 The predisposing genetic factors, in combination with internal and external influences, play a crucial role in its pathophysiology.2,3 Family and twin studies estimate the heritability of migraine to be between 30% to 60%, with migraine with aura (MA) exhibiting a stronger genetic predisposition than migraine without aura (MO).4-8 While rare monogenic subtypes, such as familial hemiplegic migraine (FHM), follow Mendelian inheritance patterns, the majority of migraine cases are polygenic, driven by numerous small-effect genetic variants interacting with environmental factors.9,10
Advancements in genomic research, particularly genome-wide association studies (GWAS) and next-generation sequencing, have significantly enhanced our understanding of migraine genetics. These studies have identified multiple susceptibility loci, reinforcing the notion that migraine is a multifactorial disorder involving both neurological and vascular components.11,12 Emerging evidence suggests that migraine spans a genetic continuum, ranging from monogenic to polygenic inheritance, rather than fitting into a strictly dichotomous classification (Figure 1).13
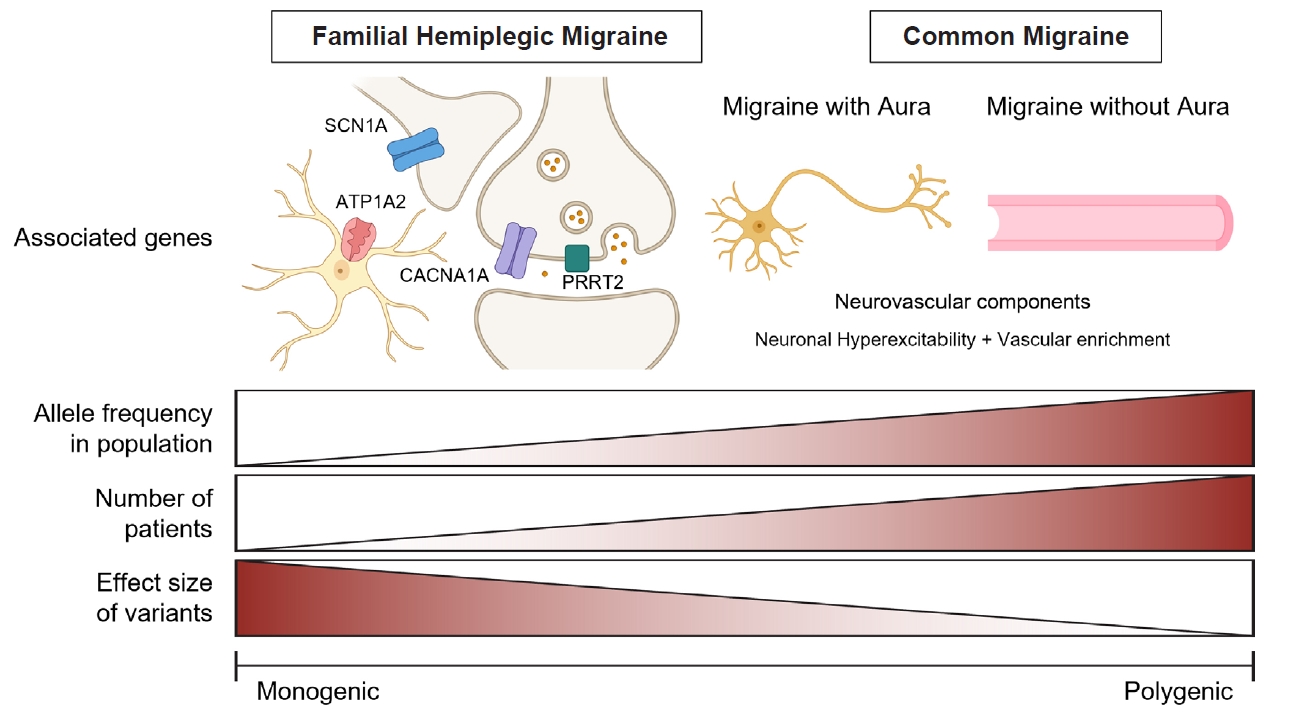
The genetic spectrum of migraine disorders from monogenic to polygenic forms. This figure illustrates the continuum of genetic architecture in migraine disorders, ranging from monogenic (left) to polygenic (right) forms. Familial hemiplegic migraine represents a monogenic form of migraine, characterized by rare, high-impact variants in genes such as CACNA1A, ATP1A2, and SCN1A. In contrast, common migraine is associated with polygenic risk factors involving genes that influence neuronal hyperexcitability and vascular components. As migraine shifts from a monogenic to a polygenic disorder, the allele frequency of associated variants in the population and the number of affected individuals increase, while the effect size of individual variants decreases. The illustration of neurons, astrocytes, and blood vessels was created using BioRender (https://BioRender.com).
Despite these advances, most genetic studies on migraine have been conducted in European populations. Heritability estimates from twin studies and findings from GWAS are predominantly based on single-ancestry cohorts, mainly of European descent.13,14 Given the differences in genetic architecture across populations, independent genetic studies in East Asian populations are essential to ensure broader applicability and to uncover population-specific genetic risk factors.15,16
This review explores recent advances in migraine genetics, covering both monogenic and polygenic forms of the disorder. Additionally, we examine the current state of genetic studies in East Asian populations. A comprehensive understanding of migraine genetics across diverse ancestries is critical for improving clinical outcomes, particularly in precision medicine.
MONOGENIC FORM OF MIGRAINE
1. Familial hemiplegic migraine
FHM is a rare monogenic subtype of migraine characterized by severe headache attacks accompanied by transient hemiparesis (motor weakness).10 The estimated prevalence of hemiplegic migraine (HM) in the Danish population is approximately 0.01%, with FHM accounting for 60% of cases and sporadic hemiplegic migraine representing the remaining 40%.17,18 FHM follows an autosomal dominant inheritance pattern with high penetrance, meaning that most individuals carrying a pathogenic mutation develop symptoms.19,20 The clinical presentation of FHM includes prolonged aura lasting from several hours to weeks, often with brainstem symptoms such as confusion, fever, coma, and seizures.1,21
The disorder is genetically heterogeneous and is primarily classified into three subtypes (FHM1, FHM2, and FHM3) caused by mutations in CACNA1A, ATP1A2, and SCN1A, respectively (Figure 1).22-24 A fourth candidate gene, PRRT2, has been proposed to be associated with FHM (FHM4), though its role remains debated.25 Additionally, recent studies suggest that mutations in other genes related to ion channel function and neurotransmission may also contribute to FHM.26 These monogenic forms of migraine provide valuable insights into the molecular mechanisms underlying migraine pathophysiology.
1) Familial hemiplegic migraine type 1
The CACNA1A gene, located on chromosome 19p13, was the first gene identified for FHM.22 It encodes the α1 subunit of the P/Q-type voltage-gated calcium channel, which is crucial for neurotransmitter release at synaptic terminals in the brain. Several CACNA1A mutations have been reported in FHM1, most of which are missense mutations resulting in a gain of function.27 These mutations lead to excessive calcium influx, increased glutamate release, and enhanced neuronal excitability, predisposing individuals to cortical spreading depression (CSD), the physiological basis of migraine aura.
FHM1 mutations are also associated with other neurological conditions, including episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6).28,29 Notably, specific mutations such as T666M and S218L are linked to more severe phenotypes, including coma and febrile seizures.30 Animal models of FHM1 have provided significant insights into its pathophysiology.31 Knock-in mice carrying the R192Q or S218L mutations show increased susceptibility to CSD, trigeminal activation, and ischemic sensitivity, further supporting the role of calcium channel dysregulation in migraine pathogenesis.32-34
2) Familial hemiplegic migraine type 2
The ATP1A2 gene, located on chromosome 1q23.2, encodes the α2 subunit of the Na+/K+-ATPase pump, which is primarily expressed in astrocytes.24 This pump is essential for maintaining ionic homeostasis and glutamate clearance from the synaptic cleft. More than 90 pathogenic mutations in ATP1A2 have been identified in FHM2, most of which result in a loss of function of the Na+/K+-ATPase pump.35 This impairment leads to extracellular potassium accumulation and impaired glutamate uptake, increasing excitability and promoting CSD.36 Notably, FHM2 accounts for the majority of FHM cases in which a pathogenic mutation has been identified.37
FHM2 is often associated with a higher incidence of epilepsy.38 Animal models have confirmed that mutations in ATP1A2 enhance susceptibility to CSD.39 Mice with a complete astrocytic knockout of ATP1A2 exhibit spontaneous waves of CSD, metabolic abnormalities, and an increased likelihood of hemiplegic attacks, supporting the role of astrocyte dysfunction in migraine.40
3) Familial hemiplegic migraine type 3
The SCN1A gene, located on chromosome 2q24.3, encodes the α1 subunit of the NaV1.1 voltage-gated sodium channel, which is highly expressed in inhibitory interneurons.23,41 Mutations in SCN1A can lead to both gain- and loss-of-function effects, altering neuronal excitability.42,43 FHM3 mutations predominantly result in gain-of-function effects, leading to increased sodium influx and hyperactivity of inhibitory interneurons. This paradoxically enhances cortical excitability, making CSD initiation easier.
Interestingly, SCN1A mutations are also strongly associated with epilepsy, particularly Dravet syndrome, further emphasizing the overlap between migraine and seizure disorders.44 Animal models carrying SCN1A mutations demonstrate increased CSD susceptibility, further supporting its role in migraine pathophysiology.45,46
2. Other monogenic form of migraine and overlapping syndromes
1) PRRT2 (Familial hemiplegic migraine type 4) and paroxysmal disorders
PRRT2 has been proposed as a fourth FHM gene, although its role remains controversial.25,47 Mutations in PRRT2 lead to haploinsufficiency and are associated with multiple neurological disorders, including benign familial infantile epilepsy, paroxysmal kinesigenic dyskinesia, and HM.48,49 PRRT2 mutations are thought to influence neuronal excitability by interacting with NaV1.2 and NaV1.6 sodium channels, as well as synaptic vesicle release machinery.50 PRRT2-knockout mice exhibit increased excitability and seizure susceptibility, suggesting that it modulates neuronal transmission in a manner relevant to migraine pathogenesis.51
2) Other monogenic migraine-related disorders
Several other monogenic disorders feature migraine as a key clinical manifestation, often alongside cerebrovascular pathology. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) is a hereditary small-vessel disease caused by mutations in NOTCH3, often presenting with MA as an early symptom.52,53 Similarly, Glucose Transporter Deficiency Syndrome (GLUT1 Deficiency), resulting from mutations in SLC2A1 (encoding GLUT1), can lead to recurrent migraine attacks due to impaired glucose transport across the blood-brain barrier.54 Additionally, several other candidate genes, including SLC4A4, ATP1A3, and SLC1A3, have been implicated in FHM-like phenotypes, although further studies are needed to confirm their causality.26,55-57
POLYGENIC FORM OF MIGRAINE
1. Migraine and other headaches
Unlike monogenic subtypes such as FHM, most migraine or headache cases are polygenic, resulting from the combined effects of multiple genetic variants, each contributing a small effect on disease susceptibility (Figure 1).9 Genetic studies, particularly GWAS, have identified numerous common variants associated with migraine risk, highlighting both neurological and vascular contributions to migraine pathophysiology.
1) Common migraine
The first significant migraine-associated locus was discovered in 2010 in a European cohort that included 2,748 migraine cases, with 429,912 single-nucleotide polymorphisms (SNPs) tested.58 Subsequent studies in 2011, 2012, and 2013 expanded the number of migraine cases and SNPs analyzed, culminating in a 2013 study that identified 12 significant loci from a dataset comprising 23,285 migraine patients and approximately 2.3 million SNPs.59-62 In 2016, Gormley et al.11 within the International Headache Genetics Consortium (IHGC) conducted a large-scale GWAS involving 59,674 migraine patients and approximately 8 million SNPs. This study identified 38 genomic loci, of which 28 were novel. These loci were significantly enriched in genes associated with arterial and smooth muscle function, aligning with prior findings of a shared polygenic risk between migraine, stroke, and cardiovascular diseases.63,64 Subsequent post-GWAS analyses revealed additional biological insights, demonstrating that neurological mechanism or metal ion homeostasis were implicated in all migraine cases, while vascular pathways were more prominently enriched in MO.65,66
Building upon this foundation, two large GWAS meta-analyses were conducted in 2021 and 2022, incorporating additional cohorts.12,67 In 2021, Choquet et al.67 performed a multi-ethnic GWAS, including individuals of East Asian, African American, and Hispanic/Latino descent. This study identified 79 independent loci significantly associated with migraine, 11 of which are not overlapped with loci in European-only studies, including Hautakangas et al.’s study.12 The following year, Hautakangas et al.12 analyzed data from 102,084 migraine patients with approximately 10 million SNPs tested. This study identified 123 genomic regions, of which 86 were novel compared to the 2016 GWAS. The findings from the 2022 GWAS reinforced the neurovascular hypothesis of migraine, with enrichment signals emerging from both central nervous system and vascular tissues. The implicated cell types play crucial roles in neuronal excitability, synaptic plasticity, neuronal development, and vascular function, further supporting the concept that migraine is a neurovascular disorder.13 Additionally, the study identified loci encoding genes that serve as established drug targets for migraine treatment. Calcitonin gene-related peptide (CGRP), encoded by CALCA/CALCB, is targeted by CGRP antibodies, while the serotonin 1F receptor is the target of ditans, a class of migraine-specific medications.68
With some risk loci exclusive to Hautakangas et al.’s12 GWAS, others found only in Choquet et al.’s study,67 and some overlapping between both studies, the number of known migraine risk loci has now expanded to 178. Furthermore, Choquet et al.67 identified three additional loci—rs1047891 (CPS1), rs11718509 (PBRM1), and rs10150336 (SLC25A21)—which were specific to women with migraine, bringing the total number of migraine-associated loci to 181.
Beyond common variants, rare variants identified through whole-genome sequencing have provided deeper insights into migraine genetics.69 The ability to detect rarer variants, down to a minor allele frequency of 0.001%, has uncovered novel loci that were previously inaccessible using GWAS alone. Some of these rare variants are enriched in genes that had already been implicated in GWAS, while others represent newly discovered risk factors. The most recent analysis identified 12 novel rare variants, spanning both non-coding regulatory regions and protein-altering mutations. Notably, the frameshift mutation in PRRT2 was strongly associated with MA and epilepsy. Additionally, rare variant analysis identified SCN11A and KCNK5 as functionally relevant genes in all migraine, suggesting the association with nociceptive processing and vascular function. These findings underscore the significance of rare variants in migraine pathophysiology and provide novel insights into the molecular mechanisms underlying disease susceptibility.
2) Migraine with and without aura
Migraine is classified into two primary subtypes: MO and MA. MA is distinguished by transient neurological symptoms, typically visual disturbances, that precede the headache phase.1 Despite extensive genetic research, early GWAS struggled to distinguish between these subtypes, as most risk variants were shared between both forms of migraine. The GWAS by Gormley et al.11 identified seven loci significantly associated with MO, including TSPAN2, TRPM8, PHACTR1, FHL5, ASTN2, FGF6, and LRP1. These loci were enriched in vascular and cardiovascular pathways, suggesting a stronger contribution of vascular mechanisms to MO compared to MA. However, this study did not identify MA-specific loci.
A more refined analysis was conducted in the GWAS by Hautakangas et al.,12 which examined genetic associations across 15,055 MO cases and 14,624 MA cases. The study identified three loci specific to MA (MPPED2, CACNA1A, and HMOX2) and two loci specific to MO (near FECH and near SPINK2), further refining the genetic distinction between the subtypes. However, in 2016, none of the seven loci originally reported in the GWAS by Gormley et al.11 were replicated in the MO subgroup analysis. CACNA1A, one of the MA-specific loci, is particularly notable as it is the causal gene for FHM1, reinforcing a genetic link between monogenic and polygenic forms of migraine.28 In 2023, the rare variant study provided further evidence supporting the genetic distinction between MA and MO.69 A rare frameshift mutation in PRRT2 was found to be strongly associated with MA but not MO, highlighting its potential role in CSD. However, while MA exhibits a stronger association with neuronal hyperexcitability, the majority of genetic risk factors are shared between MA and MO.
3) Other headache disorders
Beyond migraine, other headache disorders have also been investigated through GWAS. In 2018, a GWAS using UK Biobank data analyzed a broadly defined headache phenotype.70
This study identified 28 loci, many of which had previously been associated with migraine, suggesting substantial genetic overlap. Although the study population was likely heterogeneous in terms of headache subtypes, the presence of known migraine loci indicated that common genetic factors influence various headache disorders. For cluster headache, the large-scale GWAS was led by the International Consortium for Cluster Headache Genetics (CCG).71,72 A more recent GWAS focusing on cluster headache found FHL5, LRP1, and PLCE1, all previously associated with migraine, to be significantly associated with cluster headache, supporting a shared genetic susceptibility between these conditions.73 In addition to these overlapping loci, the study identified distinct genetic variants specific to general headache or cluster headache, indicating that while migraine, general headache, and cluster headache share some common risk factors, they also have unique genetic contributions that differentiate their pathophysiology.
2. Clinical and research applications of genome-wide association studies findings in migraine
1) Polygenic risk scores for migraine susceptibility
Polygenic risk scores (PRS) have emerged as a powerful tool to quantify the cumulative genetic burden of migraine, integrating the effects of multiple small-effect variants identified in GWAS.74 Studies have shown that familial migraine cases, particularly those with MA and FHM, exhibit higher PRS compared to non-familial cases, supporting the notion that most migraine results from the accumulation of numerous minor risk alleles rather than a single high-impact mutation.75 Notably, PRS analysis in a Finnish cohort demonstrated a correlation between genetic risk and the continuum of migraine severity, from non-migraine headaches to probable migraine, typical migraine, and HM.76 These findings suggests that genetic factors may potentially complement the current clinical framework based on the International Classification of Headache Disorders. Furthermore, PRS has been leveraged to explore pharmacogenetic applications, with studies identifying patient subgroups more likely to respond to triptans.77,78 However, its role in predicting response to preventive treatments remains inconclusive, highlighting the need for further research to establish its utility in personalized migraine management.
2) Shared genetic risk factors between migraine and other neurological disorders
Migraine shares substantial genetic overlap with various neurological, psychiatric, and vascular disorders, as demonstrated by GWAS, genetic correlation analyses, and Mendelian randomization (MR) studies.79,80 The Brainstorm Consortium,81 which analyzed over one million individuals across 25 brain disorders, found the significant associations between migraine and conditions such as major depressive disorder, attention deficit hyperactivity disorder, and Tourette’s syndrome. Additional studies have identified multiple shared loci between migraine and schizophrenia and depression, suggesting pleiotropy—where genetic variants contribute to multiple conditions.82-84 Beyond psychiatric disorders, migraine has also been genetically correlated with stroke, sleep disorders, endometriosis, fibromuscular dysplasia, type 2 diabetes, and autoimmune diseases.64,85-88 Notably, insomnia demonstrated the strongest genetic overlap with migraine, followed by short sleep duration, difficulty awakening, and daytime sleepiness.85 Genetic links between migraine and cardiovascular conditions further reinforce its neurovascular basis, with evidence suggesting that blood pressure regulation, lipid metabolism, and endothelial function play key roles in migraine pathogenesis.88,89
MR studies provide insights into potential causal relationships between migraine and its comorbid conditions.37 Multiple MR studies have investigated the relationship between migraine and stroke, and two studies found no causal link, while one suggested a protective effect of large artery stroke on migraine risk.19,90,91 MR has, however, established a causal effect of elevated blood pressure on increased migraine susceptibility, supporting the involvement of vascular dysfunction in migraine pathophysiology.88,89 Similarly, MR studies have indicated that difficulty awakening and insomnia significantly increase migraine risk, further reinforcing the bidirectional relationship between sleep regulation and migraine.92,93 Behavioral traits such as alcohol and coffee consumption were associated with a reduced risk of migraine, while smoking initiation showed a potential causal link to increased migraine susceptibility.94
CURRENT STATUS OF GENETIC STUDIES IN EAST ASIAN POPULATIONS
Given the known differences in genetic architecture across populations, independent genetic studies in diverse ancestries are essential to ensure the broader applicability of migraine research.15,16 However, most genetic studies on migraine have been conducted in individuals of European descent led by IHGC and CCG, with relatively limited data available for East Asian populations (Figure 2).95 This section explores the current status of migraine genetics research in East Asian populations, including findings from FHM studies and GWAS (Table 1).
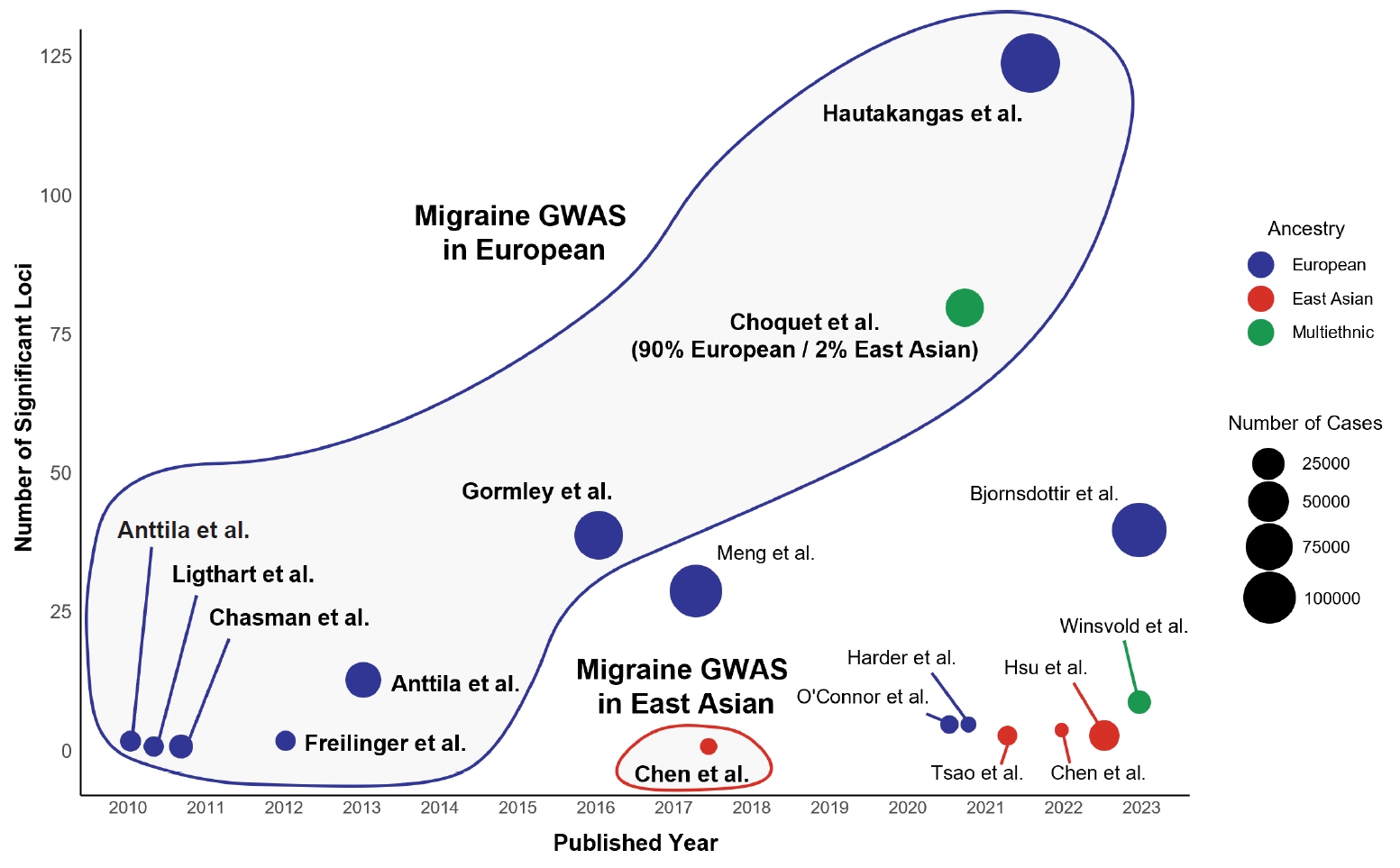
Genome-wide association studies (GWAS) for migraine and other headache disorders across European and East Asian populations. This figure presents GWAS for migraine, self-reported headache, and cluster headache across European, East Asian, and multiethnic populations. Each dot represents a GWAS, with the x-axis indicating the publication year and the y-axis showing the number of significant loci (p<5×10−8). Dot colors represent ancestry, with blue indicating European populations, red for East Asian populations, and green for multiethnic populations. The size of each dot corresponds to the number of enrolled cases in the study. The outlined clusters highlight distinct population-based GWAS trends on migraine, emphasizing the predominance of European studies and underscoring the need for further research in East Asian populations. Although Choquet et al.67 conducted a multiethnic study, more than 90% of the population was of European ancestry; therefore, it was grouped within the European migraine GWAS cluster.
1. Hemiplegic migraine cases in East Asian population
Several case studies and small cohort analyses have identified pathogenic mutations in CACNA1A, ATP1A2, and SCN1A in East Asian FHM patients. While early reports focused on individual cases in China96,97, a larger study identified 15 cases with ATP1A2 mutations, revealing 12 missense variants, including a novel variant.98 Some mutations, such as c.2143G>A (p.G715R), had been previously reported in European patients, but clinical presentations in Chinese cases exhibited distinct features, including frequent encephalopathy, developmental delay, and epilepsy. Additionally, the novel variant c.2564G>A (p.G855E) occurred at the same site as previously reported variants c.2563G>A (p.G855R) and c.2564G>T (p.G855V), suggesting a potential hotspot for pathogenic mutations. In the Korean population, two cases of FHM have been reported, including one novel variant (c.3005G>A, p.R1002Q) that had not been previously documented.99,100 In Japan, a study identified four variants in ATP1A2, three of which were novel.101
Notably, a Chinese cohort study identified novel CACNA1A mutations associated with severe FHM1 phenotypes, including ataxia and epilepsy, highlighting potential population-specific mutational profiles.102,103 Additionally, a Japanese study detected five heterozygous missense mutations in SCN1A, three of which were novel.104 While many FHM-related variants have been reported in both European and East Asian populations, a subset of mutations appears to be unique to East Asians. Compared to polygenic migraine, monogenic forms of migraine are less influenced by genetic background based on ancestry, yet certain population-specific differences still exist.
2. Genome-wide association studies in East Asian population
1) Common migraine
The earliest migraine genetic studies in East Asians primarily focused on candidate gene approaches, attempting to replicate findings from European GWAS. Two studies in Chinese populations confirmed an association between PRDM16 (rs2651899) and migraine, consistent with European findings, whereas TRPM8 (rs10166942) and LRP1 (rs11172113) did not show significant associations in the Chinese cohorts.105,106 The replication efforts in the She ethnic group showed significant associations only for C7orf10 (rs4379368) and FHL5 (rs13208321) among five candidate variants, highlighting potential population-specific differences in migraine susceptibility.107 A subsequent Chinese study analyzing 18 candidate SNPs from previous GWAS and serotonin receptor-related genes identified three significant risk loci—MEF2D (rs2274316), ASTN2 (rs6478241), and PRDM16 (rs2651899)—further supporting the relevance of previously discovered European loci in East Asian populations.108 More recently, an investigation in 2021 explored 22 pre-selected SNPs in KCNK5 and FHL5, strengthening the link between these loci and migraine risk in East Asians.109
The first independent GWAS on migraine in an East Asian population was conducted in Taiwan in 2018 (Table 1).110 This study analyzed a discovery cohort of 1,005 migraine cases and 1,053 controls, followed by replication in an additional 1,120 migraine cases and 604 controls. The study identified four novel loci such as DLG2 (rs655484) and GFRA1 (rs3781545), while also observing similar association trend at TRPM8 (rs10166942) and LRP1 (rs1172113), with European GWAS. These findings suggest that while migraine shares a common genetic basis across different populations, most risk variants may be specific to East Asians. In addition to this study, another Taiwanese research group conducted two GWAS focused on migraine subtypes rather than using typical case-control designs.111,112 A 2021 study investigated genetic variants associated with the age of migraine onset, analyzing 715 migraine patients, while a 2024 study examined familial factors and genetic risk loci in a larger cohort of 1,561 migraine patients and 473 non-migraine controls.
A multiethnic GWAS was performed in 2021, incorporating individuals of European, East Asian, African American, and Hispanic/Latino descent (Table 1).67 While this study identified 79 independent migraine-associated loci, including 11 novel ones not discovered from European GWAS, its sample size remained heavily skewed toward individuals of European ancestry, with only 569 of the 28,852 migraine cases (approximately 2.0%) being of East Asian descent. As a result, the findings from this study may not fully capture the genetic landscape of migraine in East Asian populations.
2) Other headache disorders
Beyond migraine, GWAS on self-reported headache or broadly defined headache phenotypes have also been conducted in East Asian populations using Taiwan biobank data (Table 1). In 2022, a GWAS of 2,084 East Asian patients with self-reported headache identified two significant loci, FGF23 (rs13312779) and TGFBR3 (rs10493859), had previously been implicated in the 2021 Choquet et al.67 and 2022 Hautakangas et al.12 GWAS study on migraine.113 In 2023, a large study analyzed 12,026 cases of broadly defined headache and 1,874 cases of severe headache.114 Broadly defined headache was classified based on self-reported headache symptoms, while severe headache was defined by its adverse impact on daily life. This study identified rs8072917 in RNF213 and ENDOV as significant loci for broadly defined headache and rs13272202 in RP11-1101K5.1 for severe headache. RNF213 had previously been implicated in both 2021 Choquet et al.67 and 2022 Hautakangas et al.12 GWAS studies on migraine, further suggesting that vascular dysfunction plays a critical role in headache pathophysiology across populations. For cluster headache, an independent GWAS in a Taiwanese cohort of 734 patients identified a novel risk locus, CAPN2 (rs1556780), as well as previously known loci from European GWAS, including MERTK (rs10188640) and STAB2 (rs13028839).115
While migraine exhibits distinct genetic characteristics compared to broadly defined headache and cluster headache across populations, some genetic loci identified in broadly defined headache overlap with those found in European migraine studies. This overlap may be attributed to the reliance of large-scale European GWAS on questionnaire-based migraine definitions, which likely capture a broader headache phenotype rather than strictly defined migraine cases. To enhance our understanding, future research should focus on precisely diagnosed migraine cases and larger East Asian cohorts, ensuring greater accuracy and applicability of genetic findings across diverse populations.
SUMMARY
This review examined the genetic landscape of migraine, which has historically been studied primarily in European ancestry. We specifically focused on genetic studies in East Asian populations, making this the first comprehensive summary of existing East Asian research to our knowledge. However, our review does not cover ancestry-specific genetic studies in African, Hispanic/Latino, or South Asian populations, highlighting the need for broader investigations in diverse ethnic groups.
Genetic studies have significantly advanced our understanding of migraine, identifying both monogenic and polygenic contributions to its pathophysiology. While FHM highlights the role of ion channel dysfunction in rare monogenic forms, large-scale GWAS have uncovered numerous common variants associated with vascular and neuronal pathways in polygenic migraine. Despite shared biological pathways across different ancestries, population-specific genetic variations suggest that the molecular mechanisms underlying migraine pathogenesis may differ across ancestries. Independent investigations in East Asian populations have identified unique risk loci, reinforcing the need for diverse ancestry-based studies to improve the generalizability of genetic findings.
Future research should explore the potential of ethnicity-based precision medicine in migraine diagnosis and treatment. PRS would offer a promising approach for predicting migraine susceptibility. Pharmacogenomic studies integrating genetic findings with clinical data may improve individualized treatment selection, particularly in predicting response to migraine-specific medications such as triptans or CGRP inhibitors. Advances in gene-targeted therapies, including Clustered regularly interspaced short palindromic repeats-based genome editing and small-molecule inhibitors, hold potential for future migraine treatment strategies by directly modifying pathogenic genetic variants. Integrating genomic insights into clinical practice will be crucial for developing personalized approaches to migraine prevention and management.
Notes
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: JK, MKC; Data curation: JK; Funding acquisition: MKC; Investigation: JK; Writing–original draft: JK, MKC; Writing–review & editing: JK, MKC.
CONFLICT OF INTEREST
Min Kyung Chu is the Associate Editor of Headache and Pain Research and was not involved in the review process of this article. And, he was a site investigator for a multicenter trial sponsored by Biohaven Pharmaceuticals, Allergan Korea, and Ildong Pharmaceutical Company. He has received lecture honoraria from Eli Lilly and Company, Handok-Teva, and Ildong Pharmaceutical Company over the past 24 months. The other author has no other conflicts of interest to declare.
FUNDING STATEMENT
This research was supported by a grant of the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. He received grants from the Yonsei University College of Medicine (6-2021-0229) and the Korea Health Industry Development Institute (HV22C0106) and a National Research Foundation of Korea grant from the Korean Government (MSIT; 2022R1A2C1091767).
ACKNOWLEDGMENTS
Not applicable.