Articles
- Page Path
- HOME > Headache Pain Res > Volume 26(2); 2025 > Article
-
Review Article
Genetic Architecture of Migraine: From Broad Insights to East Asian Perspectives -
Joonho Kim1,2
 , Min Kyung Chu1
, Min Kyung Chu1
-
Headache and Pain Research 2025;26(2):116-129.
DOI: https://doi.org/10.62087/hpr.2025.0003
Published online: May 27, 2025
1Department of Neurology, Yonsei University College of Medicine, Seoul, Republic of Korea
2Department of Biomedical Systems Informatics, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Republic of Korea
- Correspondence: Min Kyung Chu, M.D., Ph.D. Department of Neurology, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Republic of Korea Tel: +82-2-2228-1600, Fax: +82-2-393-0705, E-mail: chumk@yonsei.ac.kr
© 2025 The Korean Headache Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,213 Views
- 39 Download
- 1 Crossref
Abstract
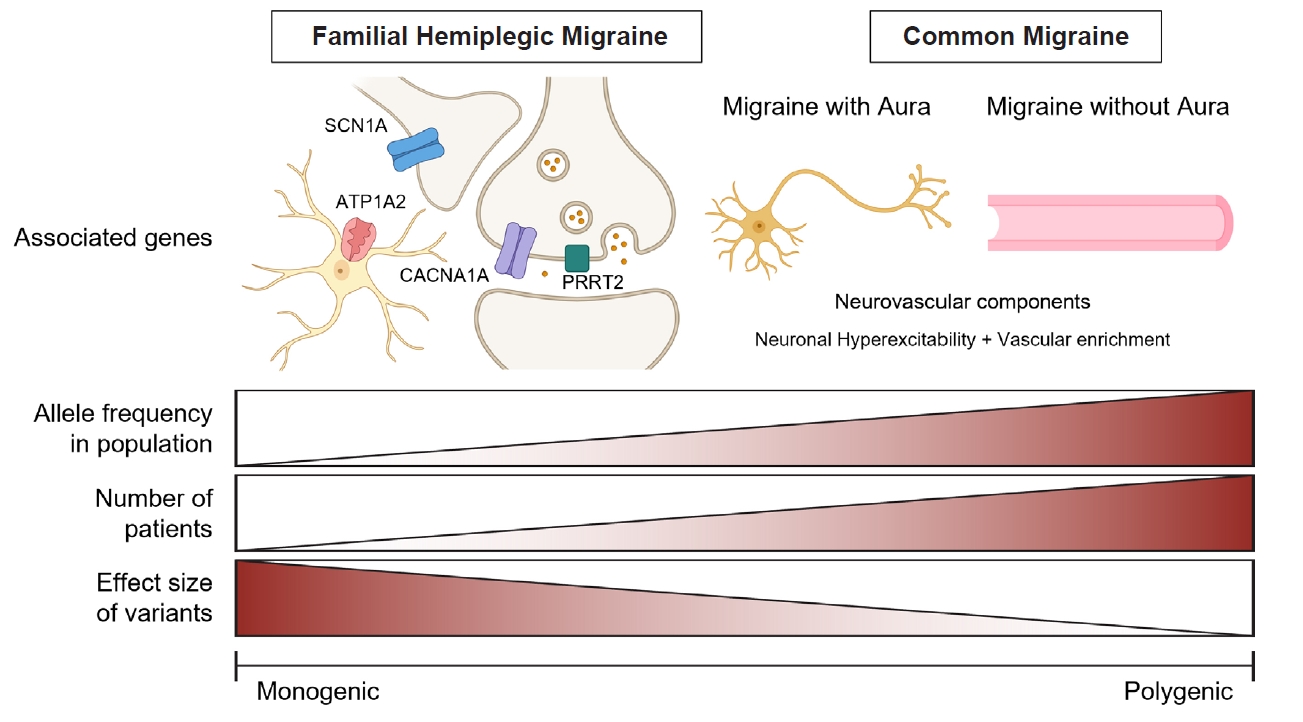
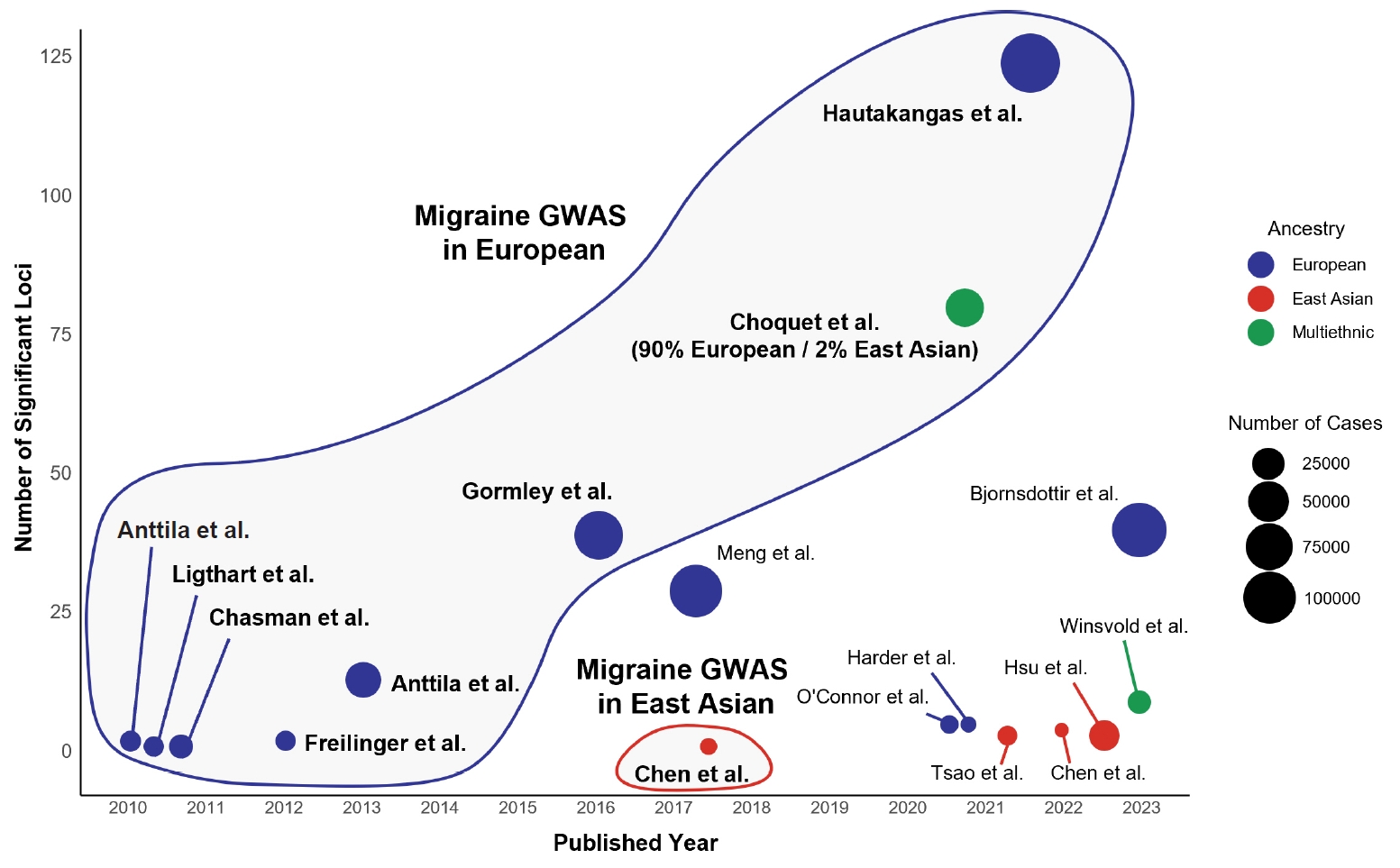
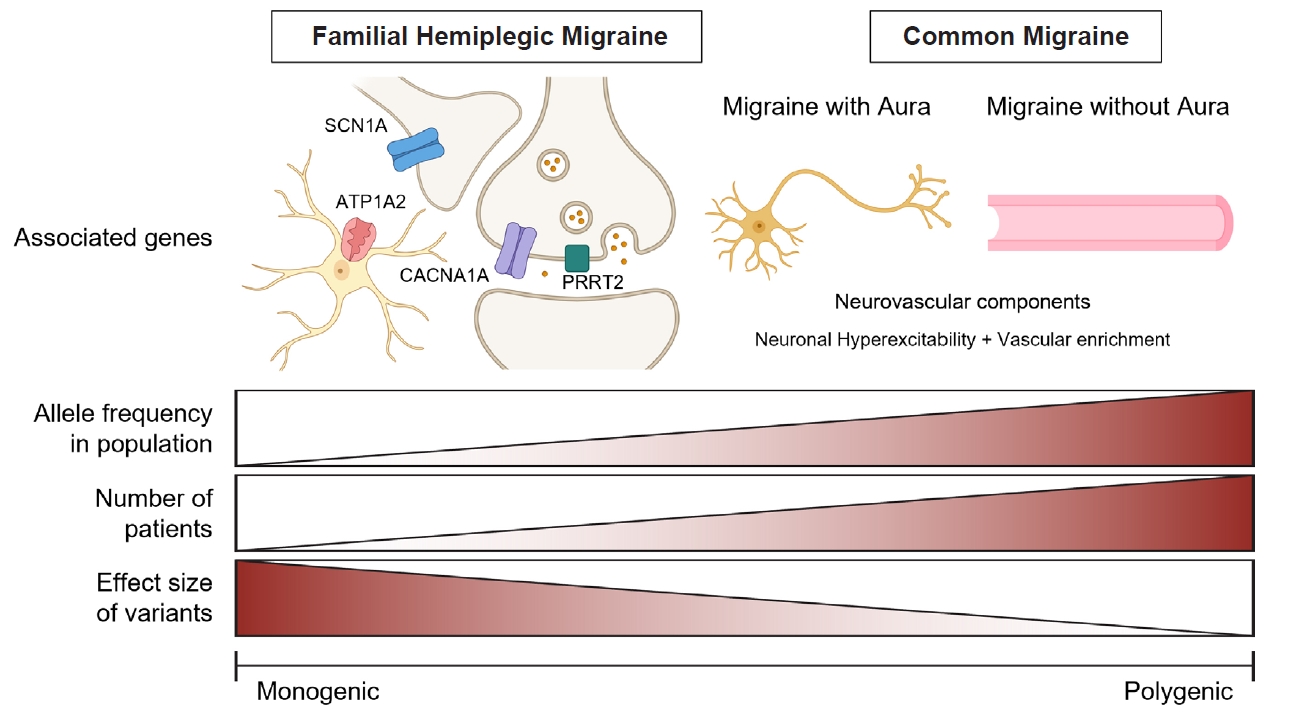
- Migraine is a complex neurological disorder with a strong genetic component, ranging from rare monogenic forms, such as familial hemiplegic migraine (FHM), to common polygenic migraine. FHM is primarily caused by mutations in CACNA1A, ATP1A2, and SCN1A, which affect ion channel function and cortical excitability. Additional genes, including PRRT2, have also been implicated, broadening the genetic landscape of monogenic migraine. Genome-wide association studies (GWAS) have identified multiple susceptibility loci for common migraine, highlighting key pathways related to neuronal excitability and vascular function. These findings have reinforced the neurovascular hypothesis of migraine pathogenesis. GWAS on other headache disorders, such as broadly defined headache or cluster headache, have also revealed both overlapping and distinct genetic risk factors. Genetic studies in East Asians have identified both ancestry-specific risk variants and overlapping loci with European populations, suggesting similarities in biological pathways while also highlighting population-specific differences in migraine susceptibility. Expanding research on the genetics of migraine in East Asian populations is essential for uncovering novel risk factors and improving the generalizability of genetic findings.
INTRODUCTION
MONOGENIC FORM OF MIGRAINE
1) Familial hemiplegic migraine type 1
2) Familial hemiplegic migraine type 2
3) Familial hemiplegic migraine type 3
1) PRRT2 (Familial hemiplegic migraine type 4) and paroxysmal disorders
2) Other monogenic migraine-related disorders
POLYGENIC FORM OF MIGRAINE
1) Common migraine
2) Migraine with and without aura
3) Other headache disorders
1) Polygenic risk scores for migraine susceptibility
2) Shared genetic risk factors between migraine and other neurological disorders
CURRENT STATUS OF GENETIC STUDIES IN EAST ASIAN POPULATIONS
1) Common migraine
2) Other headache disorders
SUMMARY
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: JK, MKC; Data curation: JK; Funding acquisition: MKC; Investigation: JK; Writing–original draft: JK, MKC; Writing–review & editing: JK, MKC.
CONFLICT OF INTEREST
Min Kyung Chu is the Associate Editor of Headache and Pain Research and was not involved in the review process of this article. And, he was a site investigator for a multicenter trial sponsored by Biohaven Pharmaceuticals, Allergan Korea, and Ildong Pharmaceutical Company. He has received lecture honoraria from Eli Lilly and Company, Handok-Teva, and Ildong Pharmaceutical Company over the past 24 months. The other author has no other conflicts of interest to declare.
FUNDING STATEMENT
This research was supported by a grant of the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. He received grants from the Yonsei University College of Medicine (6-2021-0229) and the Korea Health Industry Development Institute (HV22C0106) and a National Research Foundation of Korea grant from the Korean Government (MSIT; 2022R1A2C1091767).
ACKNOWLEDGMENTS
Not applicable.
| Article (year) | Headache type | Population origin | Case (n) | Control (n) | Tested single-nucleotide polymorphisms | Significant loci (n) | Genes with novel loci | Genes with overlapping loci‡ |
|---|---|---|---|---|---|---|---|---|
| Chen et al. (2018)110 | Migraine | East Asian (Han Chinese) | 1,005 for discovery, 1,120 for replication | 1,053 for discovery, 604 for replication | 642,832 | 4* | DLG2, GFRA1, GPR39, UPP2 | - |
| Choquet et al. (2021)67 | Migraine | East Asian (with African American, Hispanic/Latino, European descent) | 569 East Asians (2.0%) out of 28,852 | 6,619 East Asians (1.3%) out of 525,717 | Over 10,000,000 | 79† | PLEKHA1, HOXB1, IQCK, CHRNB1, ZC3H7B, C1orf122, TMEM91, MTF1, POLR2A, PRPF3, PHB | 68 Genes§ |
| Tsao et al. (2022)113 | Self-reported headache | East Asian (Taiwanese) | 2,084 | 11,822 | 653,291 | 2† | - | TGFBR3, FGF23 |
| Chen et al. (2022)115 | Cluster headache | East Asian (Han Chinese) | 734 | 9,846 | 6,630,979 | 3† | CAPN2 | MERTK, SATB2 |
| Hsu et al. (2023)114 | Broadly defined headache | East Asian (Han Chinese) | 12,026 | 96,829 | 9,809,486 | 2† | - | RNF213 |
| Severe headache | East Asian (Han Chinese) | 1,874 | 96,829 | 9,809,486 | 2† | RP11-1101K5.1 | - |
- 1. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.ArticlePubMedPDF
- 2. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol 2018;17:174-182.ArticlePubMed
- 3. Mulder EJ, Van Baal C, Gaist D, et al. Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 2003;6:422-431.ArticlePubMed
- 4. Russell MB, Hilden J, Sørensen SA, Olesen J. Familial occurrence of migraine without aura and migraine with aura. Neurology 1993;43:1369-1373.ArticlePubMed
- 5. Russell MB, Iselius L, Olesen J. Migraine without aura and migraine with aura are inherited disorders. Cephalalgia 1996;16:305-309.ArticlePubMedPDF
- 6. Russell MB, Olesen J. Increased familial risk and evidence of genetic factor in migraine. BMJ 1995;311:541-544.ArticlePubMedPMC
- 7. Polderman TJ, Benyamin B, de Leeuw CA, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 2015;47:702-709.ArticlePubMedPDF
- 8. Russell MB, Ulrich V, Gervil M, Olesen J. Migraine without aura and migraine with aura are distinct disorders. A population-based twin survey. Headache 2002;42:332-336.ArticlePubMed
- 9. Cader MZ. The genetics of migraine and the path to precision medicine. Prog Brain Res 2020;255:403-418.ArticlePubMed
- 10. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol 2011;10:457-470.ArticlePubMed
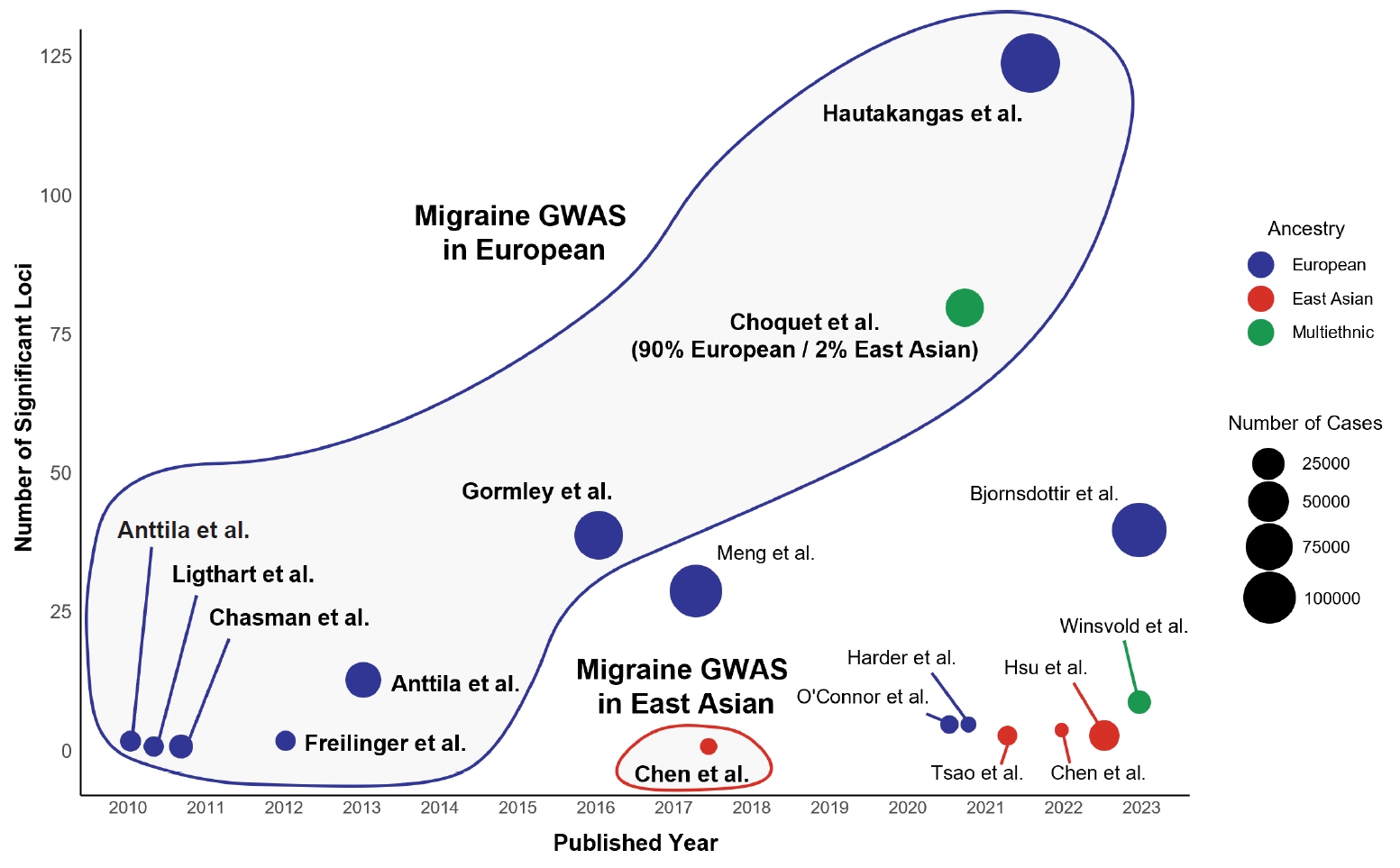
- 11. Gormley P, Anttila V, Winsvold BS, et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat Genet 2016;48:856-866.ArticlePubMedPMC
- 12. Hautakangas H, Winsvold BS, Ruotsalainen SE, et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat Genet 2022;54:152-160.ArticlePubMedPMC
- 13. Sutherland HG, Jenkins B, Griffiths LR. Genetics of migraine: complexity, implications, and potential clinical applications. Lancet Neurol 2024;23:429-446.ArticlePubMed
- 14. Olofsson IA. Migraine heritability and beyond: a scoping review of twin studies. Headache 2024;64:1049-1058.ArticlePubMed
- 15. Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019;51:584-591.ArticlePubMedPMCPDF
- 16. The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 2010;467:1061-1073.ArticlePubMedPMCPDF
- 17. Thomsen LL, Eriksen MK, Roemer SF, Andersen I, Olesen J, Russell MB. A population-based study of familial hemiplegic migraine suggests revised diagnostic criteria. Brain 2002;125:1379-1391.ArticlePubMed
- 18. Lykke Thomsen L, Kirchmann Eriksen M, Faerch Romer S, et al. An epidemiological survey of hemiplegic migraine. Cephalalgia 2002;22:361-375.ArticlePubMedPDF
- 19. Bron C, Sutherland HG, Griffiths LR. Exploring the hereditary nature of migraine. Neuropsychiatr Dis Treat 2021;17:1183-1194.ArticlePubMedPMCPDF
- 20. Sutherland HG, Albury CL, Griffiths LR. Advances in genetics of migraine. J Headache Pain 2019;20:72.ArticlePubMedPMCPDF
- 21. Thomsen LL, Ostergaard E, Olesen J, Russell MB. Evidence for a separate type of migraine with aura: sporadic hemiplegic migraine. Neurology 2003;60:595-601.ArticlePubMed
- 22. Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543-552.ArticlePubMed
- 23. Dichgans M, Freilinger T, Eckstein G, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005;366:371-377.ArticlePubMed
- 24. De Fusco M, Marconi R, Silvestri L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 2003;33:192-196.ArticlePubMedPDF
- 25. Riant F, Roos C, Roubertie A, et al. Hemiplegic migraine associated with PRRT2 variations: a clinical and genetic study. Neurology 2022;98:e51-e61.ArticlePubMed
- 26. Sutherland HG, Maksemous N, Albury CL, et al. Comprehensive exonic sequencing of hemiplegic migraine-related genes in a cohort of suspected probands identifies known and potential pathogenic variants. Cells 2020;9:2368.ArticlePubMedPMC
- 27. Tottene A, Fellin T, Pagnutti S, et al. Familial hemiplegic migraine mutations increase Ca(2+) influx through single human CaV2.1 channels and decrease maximal CaV2.1 current density in neurons. Proc Natl Acad Sci U S A 2002;99:13284-13289.ArticlePubMedPMC
- 28. Indelicato E, Boesch S. From genotype to phenotype: expanding the clinical spectrum of CACNA1A variants in the era of next generation sequencing. Front Neurol 2021;12:639994.ArticlePubMedPMC
- 29. Sintas C, Carreño O, Fernàndez-Castillo N, et al. Mutation spectrum in the CACNA1A gene in 49 patients with episodic ataxia. Sci Rep 2017;7:2514.ArticlePubMedPMCPDF
- 30. Ducros A, Denier C, Joutel A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med 2001;345:17-24.ArticlePubMed
- 31. Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 2009;61:762-773.ArticlePubMed
- 32. van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004;41:701-710.ArticlePubMed
- 33. van den Maagdenberg AM, Pizzorusso T, Kaja S, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol 2010;67:85-98.ArticlePubMed
- 34. Meneghetti N, Cerri C, Vannini E, et al. Synaptic alterations in visual cortex reshape contrast-dependent gamma oscillations and inhibition-excitation ratio in a genetic mouse model of migraine. J Headache Pain 2022;23:125.ArticlePubMedPMCPDF
- 35. Li Y, Tang W, Kang L, et al. Functional correlation of ATP1A2 mutations with phenotypic spectrum: from pure hemiplegic migraine to its variant forms. J Headache Pain 2021;22:92.ArticlePubMedPMCPDF
- 36. Capuani C, Melone M, Tottene A, et al. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med 2016;8:967-986.ArticlePubMedPMCPDF
- 37. Grangeon L, Lange KS, Waliszewska-Prosół M, et al. Genetics of migraine: where are we now? J Headache Pain 2023;24:12.ArticlePubMedPMCPDF
- 38. Friedrich T, Tavraz NN, Junghans C. ATP1A2 mutations in migraine: seeing through the facets of an ion pump onto the neurobiology of disease. Front Physiol 2016;7:239.ArticlePubMedPMC
- 39. Leo L, Gherardini L, Barone V, et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet 2011;7:e1002129.ArticlePubMedPMC
- 40. Smith SE, Chen X, Brier LM, et al. Astrocyte deletion of α2-Na/K ATPase triggers episodic motor paralysis in mice via a metabolic pathway. Nat Commun 2020;11:6164.ArticlePubMedPMCPDF
- 41. Martin MS, Dutt K, Papale LA, et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem 2010;285:9823-9834.ArticlePubMedPMC
- 42. Cestèle S, Scalmani P, Rusconi R, Terragni B, Franceschetti S, Mantegazza M. Self-limited hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci 2008;28:7273-7283.ArticlePubMedPMC
- 43. Bertelli S, Barbieri R, Pusch M, Gavazzo P. Gain of function of sporadic/familial hemiplegic migraine-causing SCN1A mutations: use of an optimized cDNA. Cephalalgia 2019;39:477-488.ArticlePubMedPDF
- 44. Ding J, Li X, Tian H, et al. SCN1A mutation-beyond dravet syndrome: a systematic review and narrative synthesis. Front Neurol 2021;12:743726.ArticlePubMedPMC
- 45. Jansen NA, Dehghani A, Linssen MML, Breukel C, Tolner EA, van den Maagdenberg AMJM. First FHM3 mouse model shows spontaneous cortical spreading depolarizations. Ann Clin Transl Neurol 2020;7:132-138.ArticlePubMedPMCPDF
- 46. Auffenberg E, Hedrich UB, Barbieri R, et al. Hyperexcitable interneurons trigger cortical spreading depression in an Scn1a migraine model. J Clin Invest 2021;131:e142202.ArticlePubMedPMC
- 47. Riant F, Roze E, Barbance C, et al. PRRT2 mutations cause hemiplegic migraine. Neurology 2012;79:2122-2124.ArticlePubMed
- 48. Cloarec R, Bruneau N, Rudolf G, et al. PRRT2 links infantile convulsions and paroxysmal dyskinesia with migraine. Neurology 2012;79:2097-2103.ArticlePubMedPMC
- 49. Gardiner AR, Bhatia KP, Stamelou M, et al. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology 2012;79:2115-2121.ArticlePubMedPMC
- 50. Fruscione F, Valente P, Sterlini B, et al. PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain 2018;141:1000-1016.ArticlePubMedPMC
- 51. Michetti C, Castroflorio E, Marchionni I, et al. The PRRT2 knockout mouse recapitulates the neurological diseases associated with PRRT2 mutations. Neurobiol Dis 2017;99:66-83.ArticlePubMedPMC
- 52. Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996;383:707-710.ArticlePubMedPDF
- 53. Tan RY, Markus HS. CADASIL: migraine, encephalopathy, stroke and their inter-relationships. PLoS One 2016;11:e0157613.ArticlePubMedPMC
- 54. Weller CM, Leen WG, Neville BG, et al. A novel SLC2A1 mutation linking hemiplegic migraine with alternating hemiplegia of childhood. Cephalalgia 2015;35:10-15.ArticlePubMedPDF
- 55. Vetro A, Nielsen HN, Holm R, et al. ATP1A2- and ATP1A3-associated early profound epileptic encephalopathy and polymicrogyria. Brain 2021;144:1435-1450.ArticlePubMedPDF
- 56. Gil-Perotín S, Jaijo T, Verdú AG, et al. Epilepsy, status epilepticus, and hemiplegic migraine coexisting with a novel SLC4A4 mutation. Neurol Sci 2021;42:3647-3654.ArticlePubMedPDF
- 57. Paucar M, Granberg T, Lagerstedt-Robinson K, et al. SLC1A3 variant associated with hemiplegic migraine and acetazolamide-responsive MRS changes. Neurol Genet 2020;6:e474.ArticlePubMedPMC
- 58. Anttila V, Stefansson H, Kallela M, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet 2010;42:869-873.ArticlePubMedPMCPDF
- 59. Ligthart L, de Vries B, Smith AV, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur J Hum Genet 2011;19:901-907.ArticlePubMedPMCPDF
- 60. Anttila V, Winsvold BS, Gormley P, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet 2013;45:912-917.ArticlePubMedPMC
- 61. Chasman DI, Schürks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet 2011;43:695-698.ArticlePubMedPMCPDF
- 62. Freilinger T, Anttila V, de Vries B, et al. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet 2012;44:777-782.ArticlePubMedPMC
- 63. Winsvold BS, Nelson CP, Malik R, et al. Genetic analysis for a shared biological basis between migraine and coronary artery disease. Neurol Genet 2015;1:e10.ArticlePubMedPMC
- 64. Malik R, Freilinger T, Winsvold BS, et al. Shared genetic basis for migraine and ischemic stroke: a genome-wide analysis of common variants. Neurology 2015;84:2132-2145.ArticlePubMedPMC
- 65. Finucane HK, Reshef YA, Anttila V, et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat Genet 2018;50:621-629.ArticlePubMedPMCPDF
- 66. Nyholt DR, Borsook D, Griffiths LR. Migrainomics - identifying brain and genetic markers of migraine. Nat Rev Neurol 2017;13:725-741.ArticlePubMedPDF
- 67. Choquet H, Yin J, Jacobson AS, et al. New and sex-specific migraine susceptibility loci identified from a multiethnic genome-wide meta-analysis. Commun Biol 2021;4:864.ArticlePubMedPMCPDF
- 68. de Vries T, Villalón CM, MaassenVanDenBrink A. Pharmacological treatment of migraine: CGRP and 5-HT beyond the triptans. Pharmacol Ther 2020;211:107528.ArticlePubMed
- 69. Bjornsdottir G, Chalmer MA, Stefansdottir L, et al. Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura. Nat Genet 2023;55:1843-1853.ArticlePubMedPMC
- 70. Meng W, Adams MJ, Hebert HL, Deary IJ, McIntosh AM, Smith BH. A genome-wide association study finds genetic associations with broadly-defined headache in UK biobank (N=223,773). EBioMedicine 2018;28:180-186.ArticlePubMedPMC
- 71. Harder AVE, Winsvold BS, Noordam R, et al. Genetic susceptibility loci in genomewide association study of cluster headache. Ann Neurol 2021;90:203-216.ArticlePubMedPMCPDF
- 72. O’Connor E, Fourier C, Ran C, et al. Genome-wide association study identifies risk loci for cluster headache. Ann Neurol 2021;90:193-202.ArticlePubMed
- 73. Winsvold BS, Harder AVE, Ran C, et al. Cluster headache genomewide association study and meta-analysis identifies eight loci and implicates smoking as causal risk factor. Ann Neurol 2023;94:713-726.ArticlePubMedPMC
- 74. Uffelmann E, Huang QQ, Munung NS, et al. Genome-wide association studies. Nat Rev Methods Primers 2021;1:59.ArticlePDF
- 75. Gormley P, Kurki MI, Hiekkala ME, et al. Common variant burden contributes to the familial aggregation of migraine in 1,589 Families. Neurol 2018;98(4):743-753.e4.Article
- 76. Häppölä P, Gormley P, Nuottamo ME, et al. Polygenic risk provides biological validity for the ICHD-3 criteria among Finnish migraine families. Cephalalgia 2022;42:345-356.ArticlePubMedPMCPDF
- 77. Chalmer MA, Esserlind AL, Olesen J, Hansen TF. Polygenic risk score: use in migraine research. J Headache Pain 2018;19:29.ArticlePubMedPMCPDF
- 78. Kogelman LJA, Esserlind AL, Francke Christensen A, et al. Migraine polygenic risk score associates with efficacy of migraine-specific drugs. Neurol Genet 2019;5(6):e364.ArticlePubMedPMC
- 79. Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 2016;48:709-717.ArticlePubMedPMCPDF
- 80. Kraft P, Chen H, Lindström S. The use of genetic correlation and Mendelian Randomization studies to increase our understanding of relationships between complex traits. Curr Epidemiol Rep 2020;7:104-112.ArticlePubMedPMCPDF
- 81. Brainstorm Consortium. Analysis of shared heritability in common disorders of the brain. Science 2018;360:eaap8757.ArticlePubMedPMC
- 82. Yang Y, Zhao H, Boomsma DI, et al. Molecular genetic overlap between migraine and major depressive disorder. Eur J Hum Genet 2018;26:1202-1216.ArticlePubMedPMCPDF
- 83. Bahrami S, Hindley G, Winsvold BS, et al. Dissecting the shared genetic basis of migraine and mental disorders using novel statistical tools. Brain 2022;145:142-153.ArticlePubMedPMCPDF
- 84. Pisanu C, Lundin E, Preisig M, et al. Major depression subtypes are differentially associated with migraine subtype, prevalence and severity. Cephalalgia 2020;40:347-356.ArticlePubMedPDF
- 85. Daghlas I, Vgontzas A, Guo Y, Chasman DI; International Headache Genetics Consortium; Saxena R. Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol 2020;7:2370-2380.ArticlePubMedPMCPDF
- 86. Adewuyi EO, Sapkota Y; International Endogene Consortium (IEC); et al. Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes (Basel) 2020;11(3):268.ArticlePubMedPMC
- 87. Georges A, Yang ML, Berrandou TE, et al. Genetic investigation of fibromuscular dysplasia identifies risk loci and shared genetics with common cardiovascular diseases. Nat Commun 2021;12:6031.ArticlePubMedPMC
- 88. Siewert KM, Klarin D, Damrauer SM, et al. Cross-trait analyses with migraine reveal widespread pleiotropy and suggest a vascular component to migraine headache. Int J Epidemiol 2020;49:1022-1031.ArticlePubMedPMC
- 89. Guo Y, Rist PM, Daghlas I, et al. A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun 2020;11:3368.ArticlePubMedPMC
- 90. Shu MJ, Li JR, Zhu YC, Shen H. Migraine and ischemic stroke: a Mendelian randomization study. Neurol Ther 2022;11:237-246.ArticlePubMedPMCPDF
- 91. Daghlas I, Sargurupremraj M, Danning R, et al. Migraine, stroke, and cervical arterial dissection: shared genetics for a triad of brain disorders with vascular involvement. Neurol Genet 2022;8:e653.ArticlePubMedPMC
- 92. Vgontzas A, Pavlović JM. Sleep disorders and migraine: review of literature and potential pathophysiology mechanisms. Headache 2018;58:1030-1039.ArticlePubMedPMCPDF
- 93. Chu S, Wu Z, Wu Z, Wu J, Qian Y. Association between insomnia and migraine risk: a case-control and bidirectional Mendelian randomization study. Pharmgenomics Pers Med 2021;14:971-976.ArticlePubMedPMCPDF
- 94. Yuan S, Daghlas I, Larsson SC. Alcohol, coffee consumption, and smoking in relation to migraine: a bidirectional Mendelian randomization study. Pain 2022;163:e342-e348.ArticlePubMed
- 95. Kim J, Chu MK. Genome-wide architecture of East Asian patients with migraine: a genome-wide association study based on familial history. J Clin Neurol 2024;20:351-352.ArticlePubMedPMCPDF
- 96. Tang W, Zhang M, Qiu E, et al. A Chinese family with familial hemiplegic migraine type 2 due to a novel missense mutation in ATP1A2. Cephalalgia 2019;39:1382-1395.ArticlePubMedPDF
- 97. Chen H, Sun X, Wang R, et al. A case report of atypical hemiplegic migraine with nonheadache onset in a Chinese child. BMC Neurol 2021;21:267.ArticlePubMedPMCPDF
- 98. Dai L, Ding C, Tian X, et al. The clinical spectrum associated with ATP1A2 variants in Chinese pediatric patients. Brain Dev 2023;45:422-431.ArticlePubMed
- 99. Kim J, Yum J, Kim N, Shin S, Chu MK. Sporadic hemiplegic migraine presenting ATP1A2 mutation in Korea. J Korean Neurol Assoc 2022;40:51-54.ArticlePDF
- 100. Lee K, Koh S, Lee JY, Kim TJ. First report of a Korean family with familial hemiplegic migraine. Korean J Headache 2023;24:24-27.PDF
- 101. Oda I, Danno D, Saigoh K, et al. Hemiplegic migraine type 2 with new mutation of the ATP1A2 gene in Japanese cases. Neurosci Res 2022;180:83-89.ArticlePubMed
- 102. Zhang L, Wen Y, Zhang Q, et al. CACNA1A gene variants in eight Chinese patients with a wide range of phenotypes. Front Pediatr 2020;8:577544.ArticlePubMedPMC
- 103. Yuan X, Zheng Y, Gao F, Sun W, Wang Z, Zhao G. Case report: a novel CACNA1A mutation caused flunarizine-responsive type 2 episodic ataxia and hemiplegic migraine with abnormal MRI of cerebral white matter. Front Neuro 2022;13:899813.ArticlePubMedPMC
- 104. Danno D, Tada H, Oda I, et al. Expanding the genetic and clinical spectrum of SCN1A-related hemiplegic migraine: analysis of mutations in Japanese. Int J Mol Sci 2025;26:1426.ArticlePubMedPMC
- 105. An XK, Ma QL, Lin Q, Zhang XR, Lu CX, Qu HL. PRDM16 rs2651899 variant is a risk factor for Chinese common migraine patients. Headache 2013;53:1595-1601.ArticlePubMed
- 106. Fan X, Wang J, Fan W, et al. Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache 2014;54:709-715.ArticlePubMed
- 107. Lin QF, Fu XG, Yao LT, et al. Association of genetic loci for migraine susceptibility in the she people of China. J Headache Pain 2015;16:553.ArticlePubMedPMCPDF
- 108. An XK, Fang J, Yu ZZ, et al. Multilocus analysis reveals three candidate genes for Chinese migraine susceptibility. Clin Genet 2017;92:143-149.ArticlePubMedPDF
- 109. Jiang Z, Zhao L, Zhang X, Zhang W, Feng Y, Li T. Common variants in KCNK5 and FHL5 genes contributed to the susceptibility of migraine without aura in Han Chinese population. Sci Rep 2021;11:6807.ArticlePubMedPMCPDF
- 110. Chen SP, Fuh JL, Chung MY, et al. Genome-wide association study identifies novel susceptibility loci for migraine in Han Chinese resided in Taiwan. Cephalalgia 2018;38:466-475.ArticlePubMedPDF
- 111. Tsai CK, Liang CS, Lin GY, et al. Identifying genetic variants for age of migraine onset in a Han Chinese population in Taiwan. J Headache Pain 2021;22:89.ArticlePubMedPMCPDF
- 112. Liu Y, Yeh PK, Lin YK, et al. Genetic risk loci and familial associations in migraine: a genome-wide association study in the Han Chinese population of Taiwan. J Clin Neurol 2024;20:439-449.ArticlePubMedPMCPDF
- 113. Tsao YC, Wang SJ, Hsu CL, et al. Genome-wide association study reveals susceptibility loci for self-reported headache in a large community-based Asian population. Cephalalgia 2022;42:229-238.ArticlePubMedPDF
- 114. Hsu WT, Lee YT, Tan J, et al. Genome-phenome wide association study of broadly defined headache. Brain Commun 2023;5:fcad167.ArticlePubMedPMCPDF
- 115. Chen SP, Hsu CL, Wang YF, et al. Genome-wide analyses identify novel risk loci for cluster headache in Han Chinese residing in Taiwan. J Headache Pain 2022;23:147.ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Toward Precision Migraine Care: Genetics, Symptoms, and Big-Data-Driven Approaches
Soo-Jin Cho
Headache and Pain Research.2025; 26(3): 171. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
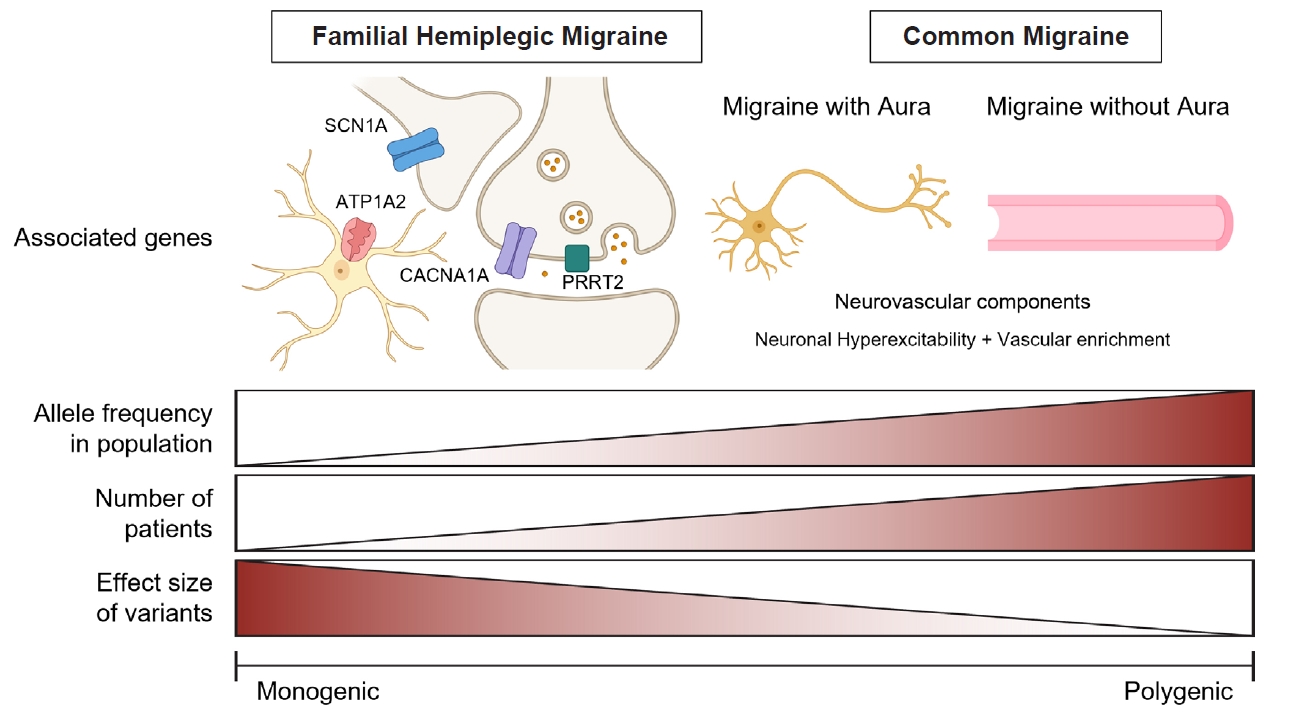
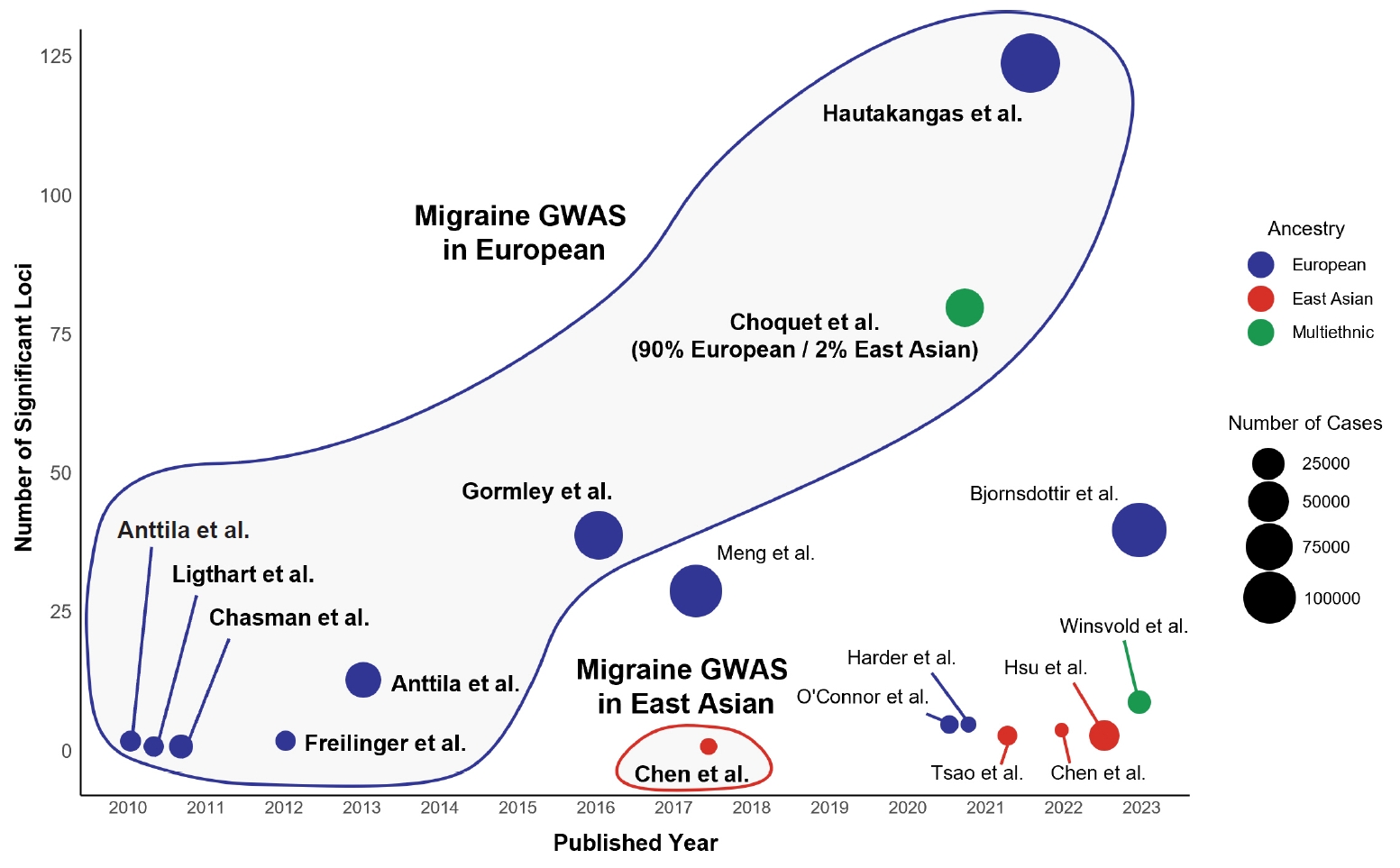
Figure 1.
Figure 2.
| Article (year) | Headache type | Population origin | Case (n) | Control (n) | Tested single-nucleotide polymorphisms | Significant loci (n) | Genes with novel loci | Genes with overlapping loci |
|---|---|---|---|---|---|---|---|---|
| Chen et al. (2018)110 | Migraine | East Asian (Han Chinese) | 1,005 for discovery, 1,120 for replication | 1,053 for discovery, 604 for replication | 642,832 | 4 |
DLG2, GFRA1, GPR39, UPP2 | - |
| Choquet et al. (2021)67 | Migraine | East Asian (with African American, Hispanic/Latino, European descent) | 569 East Asians (2.0%) out of 28,852 | 6,619 East Asians (1.3%) out of 525,717 | Over 10,000,000 | 79† | PLEKHA1, HOXB1, IQCK, CHRNB1, ZC3H7B, C1orf122, TMEM91, MTF1, POLR2A, PRPF3, PHB | 68 Genes |
| Tsao et al. (2022)113 | Self-reported headache | East Asian (Taiwanese) | 2,084 | 11,822 | 653,291 | 2 |
- | TGFBR3, FGF23 |
| Chen et al. (2022)115 | Cluster headache | East Asian (Han Chinese) | 734 | 9,846 | 6,630,979 | 3 |
CAPN2 | MERTK, SATB2 |
| Hsu et al. (2023)114 | Broadly defined headache | East Asian (Han Chinese) | 12,026 | 96,829 | 9,809,486 | 2 |
- | RNF213 |
| Severe headache | East Asian (Han Chinese) | 1,874 | 96,829 | 9,809,486 | 2 |
RP11-1101K5.1 | - |
Significant association with p-values <5×10−4. Significant association with p-values <5×10−8. Genes overlapping with European genome-wide association studies findings. All gene names were not specified in this table.
Table 1.
TOP
 KHS
KHS