Trigeminal Autonomic Cephalalgias Following Unilateral Dorsolateral Medullary Infarction: A Case Series and Literature Review
Article information
Abstract
Purpose
Secondary trigeminal autonomic cephalalgias (TACs) are typically associated with posterior fossa abnormalities, such as tumors and vascular malformations. However, TACs following brainstem infarctions are rarely reported. This study aimed to characterize the clinical and anatomical features of TACs after unilateral dorsolateral medullary infarction.
Methods
We analyzed four patients with dorsolateral medullary infarction who developed secondary TACs, diagnosed using the International Classification of Headache Disorders, third edition criteria. All patients underwent detailed neurological examinations and neuroimaging, including diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Additionally, five published cases were identified through a literature review and analyzed in conjunction with our cohort.
Results
All patients exhibited stabbing or electric shock-like pain in the ipsilateral periorbital, hemifacial, and temporal regions. Headaches developed weeks to months post-stroke with brief attacks (1–2 minutes) occurring 1–5 times daily. Lacrimation and conjunctival injection were common. Three patients were diagnosed with short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT), while a fourth had short-lasting unilateral neuralgiform with cranial autonomic symptoms (SUNA). Each patient, as well as four of the five from the literature, exhibited ipsilateral facial sensory loss, suggesting involvement of the trigeminal spinal tract and nucleus. Delayed headache onset was more frequent in persistent cases.
Conclusion
Headache characteristics were more consistent with SUNCT/SUNA than with typical cluster headaches. Careful neurological examination is essential to detect focal signs and guide neuroimaging for identifying secondary causes. Clinicians should consider secondary TACs in patients with new-onset SUNCT/SUNA and focal brainstem signs.
INTRODUCTION
Trigeminal autonomic cephalalgias (TACs) are a group of primary headache syndromes characterized by severe, short-lasting, unilateral headaches accompanied by paroxysmal cranial autonomic symptoms.1 Although several cases of secondary TACs have been reported, a definitive causal relationship between the underlying pathophysiology and the associated structural lesion in most cases remains uncertain.2,3 Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) and short-lasting unilateral neuralgiform with cranial autonomic symptoms (SUNA) are categorized under TACs and are typically considered primary headache disorders.4 Although rare, secondary causes, including structural lesions such as neoplasms, vascular malformations, demyelinating plaques, and infarctions, have been documented, particularly in patients with atypical features or poor treatment response.4,5 A comprehensive review by Cao et al.5 reported that approximately 15%–20% of SUNCT/SUNA cases may also present a secondary etiology, most commonly involving lesions in the pons, medulla, or cavernous sinus. According to Kang and Cho,4 structural abnormalities have been observed in some patients with SUNCT or SUNA, especially in those presenting with neurological signs or unusual headache characteristics. These findings underscore the importance of neuroimaging and detailed neurological examinations in patients with SUNCT/SUNA to identify potential secondary causes that may significantly influence diagnosis, management, and prognosis.
However, reports of secondary TACs remain limited, and additional case-based observations are needed to provide a more comprehensive understanding of the underlying mechanisms and clinical course.4,6-9 Thus, this study represents a case series of patients with secondary TACs following unilateral dorsolateral medullary infarction, aimed at clarifying the clinical characteristics and anatomical substrates of this patient subset.
MATERIALS AND METHODS
1. Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Chonnam National University Hospital (IRB No. CNUH-2025-258), and all patients provided written informed consent for publication.
2. Participants and procedures
The study subjects were consecutively enrolled from patients who visited Chonnam National University Hospital between January 2015 and April 2025. The inclusion criteria in this study were as follows: 1) a unilateral high signal intensity lesion involving the dorsolateral medulla suggestive of cerebral infarction on brain magnetic resonance imaging (MRI) including diffusion-weighted imaging (DWI); 2) a headache that developed after the index infarction, fulfilling the diagnostic criteria of TACs according to the International Classification of Headache Disorders, third edition (ICHD-3). Patients with a prior history of chronic headache, underlying structural brain lesions unrelated to infarction, or incomplete imaging data were excluded.
All patients underwent emergency brain MRI on admission as part of the institutional acute stroke protocol. The MRI protocol consisted of DWI (slice thickness, 4 mm), fluid-attenuated inversion recovery, gradient echo imaging, and time-of-flight magnetic resonance angiography (MRA) sequentially.
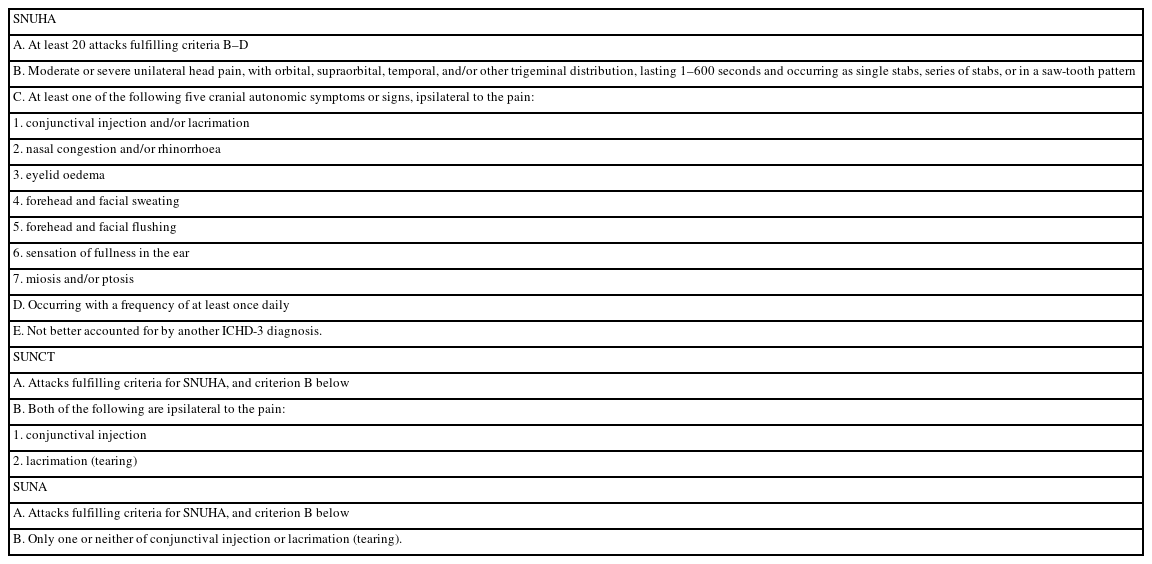
Additionally, all patients received detailed neurological examinations at the time of the initial event. Baseline demographics and headache characteristics, including timing of onset, frequency, duration, location, quality, and associated cranial autonomic features, were systematically evaluated through clinical interviews and chart reviews. SUNCT or SUNA diagnoses were performed in accordance with the ICHD-3 criteria (Table 1).
3. Data collection
To identify previously reported cases of secondary SUNCT/SUNA associated with dorsolateral medullary infarction, we searched the PubMed and MEDLINE databases up to April 2025 using the terms “SUNCT,” “SUNA,” “secondary,” and “medullary infarction.” Inclusion was limited to peer-reviewed case reports or case series with radiologically confirmed medullary infarcts and headache features consistent with TACs.
RESULTS
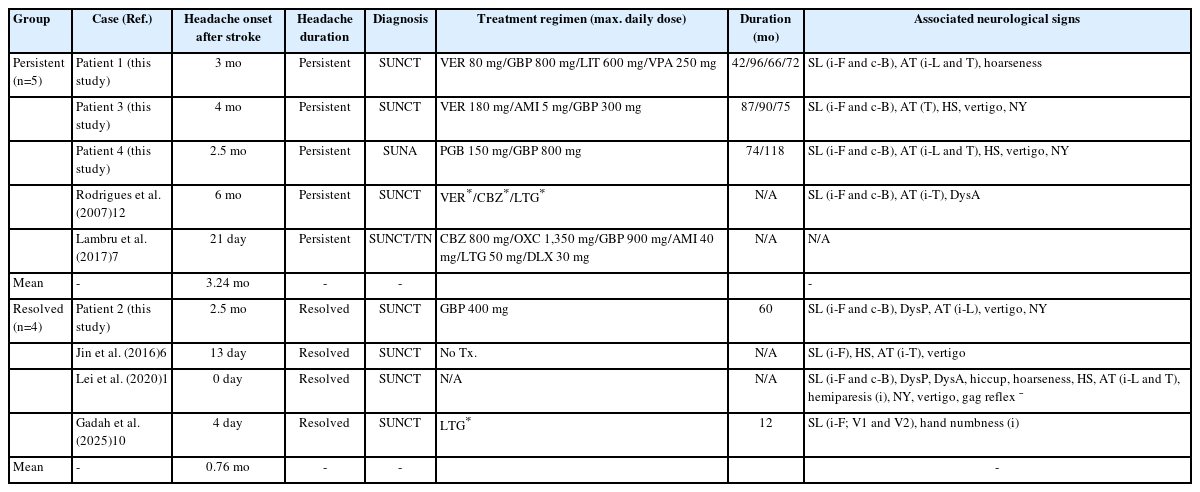
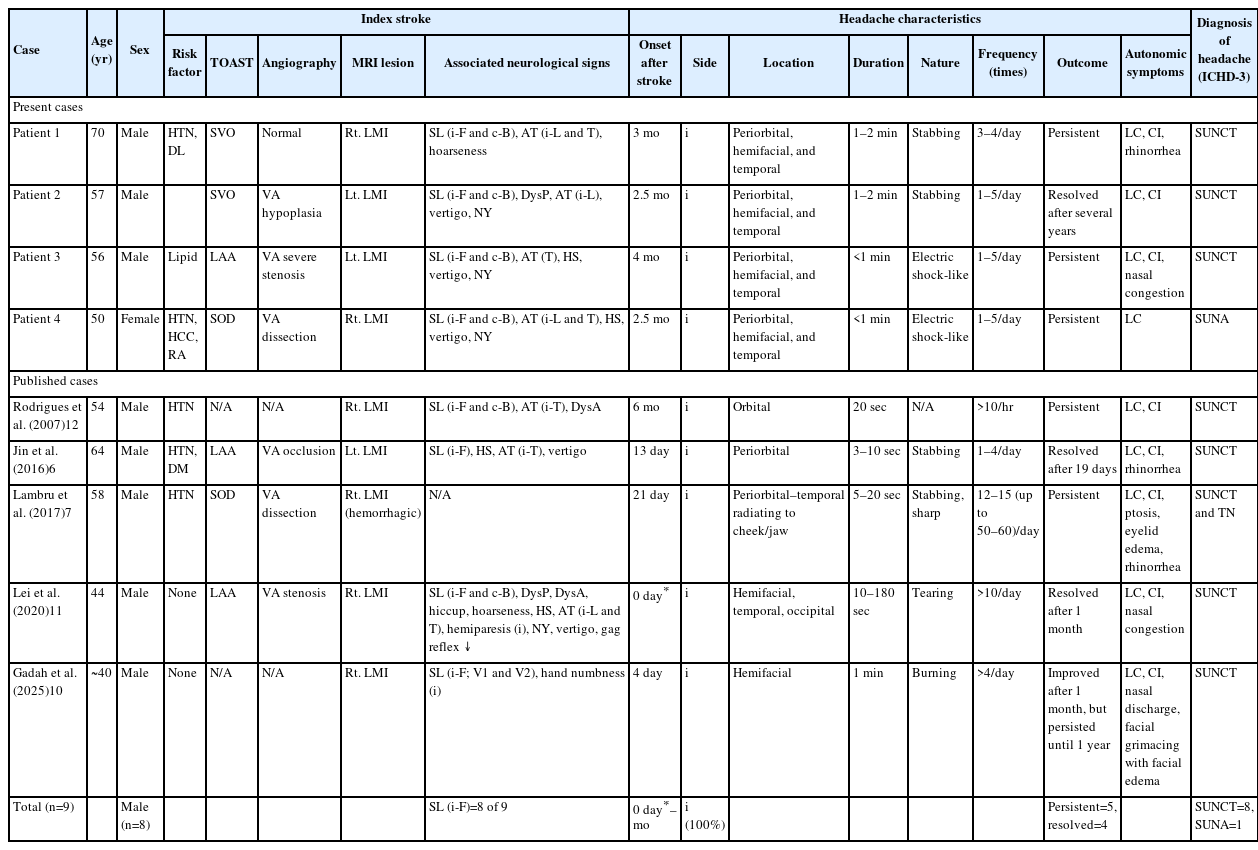
Among the 231 patients diagnosed with dorsolateral medullary infarction during the study period, 204 completed at least 6 months of follow-up after the index stroke and were eligible for analysis. Of these, four patients (three males; mean age, 58.3±8.4 years; 4/204 [1.96%]) developed headache syndromes consistent with TACs and were included in this study. Baseline demographics, characteristics of the index stroke, MRI-identified lesions, and headache characteristics are summarized in Table 2 and Figure 1. All patients exhibited either stabbing (50%) or electric shock-like pain in the ipsilateral periorbital, hemifacial, and temporal regions. Headaches developed 2.5 to 4 months after the initial infarction (mean, 3 months) with brief attacks lasting 1–2 minutes, occurring 1–5 times daily. Lacrimation (100%) and conjunctival injection (75%) were common cranial autonomic symptoms, all of which were observed on the ipsilateral side. According to the diagnostic criteria of ICHD-3, three patients (75%) were compatible with a SUNCT diagnosis, while one experienced SUNA, rather than a typical trigeminal neuralgia (TN) or cluster headache. The headaches persisted for several years in three patients (75%) despite empirical treatment with medications including gabapentin, amitriptyline, or lithium.
Clinical characteristics of TACs secondary to dorsolateral medullary infarction: present cases and literature review
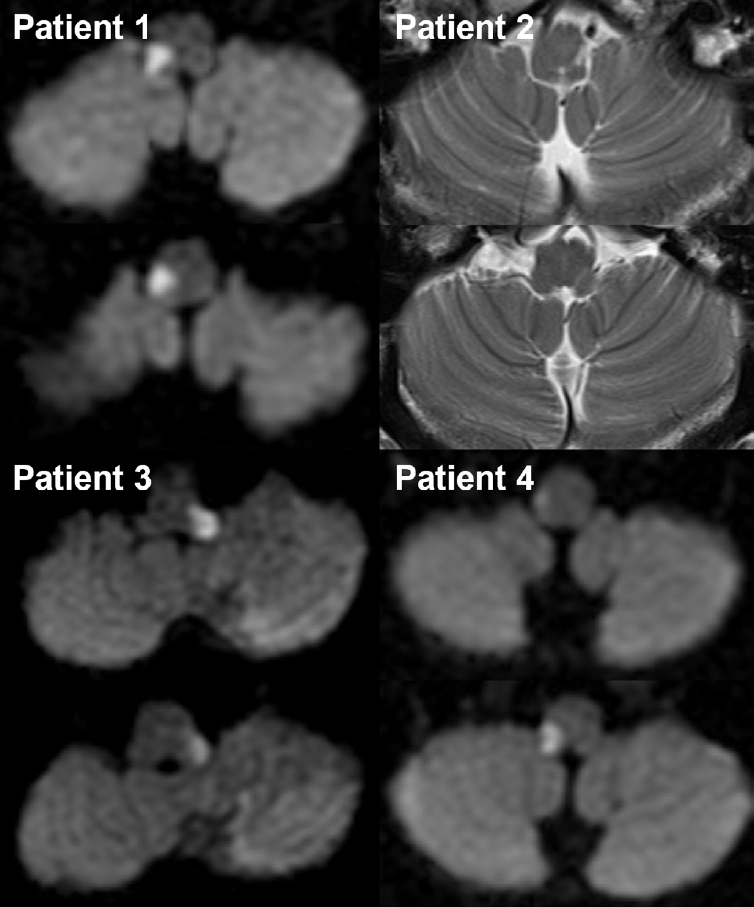
Neuroimaging of the four patients who presented with dorsolateral medullary infarction.
Diffusion-weighted (patients 1, 3, and 4) or T2-weighted (patient 2) brain magnetic resonance imaging shows high signal intensity lesions involving the unilateral dorsolateral medulla. An additional lesion in the ipsilateral cerebellar hemisphere is also observed in patient 3.
Notably, all patients showed at least one focal neurological sign during the acute stroke phase, which preceded the onset of the headache. These included sensory loss on the ipsilateral hemiface (100%), sensory loss on the contralateral hemibody, ipsilateral limb ataxia and/or truncal ataxia, and spontaneous horizontal and torsional nystagmus beating toward the contralesional side. Based on the neuroimaging results of the patients, a clinico-anatomical correlation analysis invariably revealed the involvement of the trigeminal spinal tract and nucleus, as well as the adjacent spinothalamic tract, inferior cerebellar peduncle, and vestibular nucleus.
Stroke mechanism analysis using the Trial of Org 10172 in Acute Stroke Treatment classification revealed small vessel occlusion in two patients, large artery atherosclerosis in one patient, and stroke of other determined etiology (i.e., vertebral artery [VA] dissection) in one patient. MRA findings varied: one patient had VA stenosis, one had VA hypoplasia, one had VA dissection, while another showed no vascular abnormality (Table 2).
From a literature review, we identified five additional published cases of secondary SUNCT/SUNA associated with dorsolateral medullary infarction (Table 2).6,7,10-12 Compared to our case series, the onset of headache in these cases varied, ranging from the day of stroke to 6 months later (mean, 1.43 months), indicating considerable heterogeneity.6,7,10-12 In three cases, the frequency of pain exceeded 10 episodes per day.7,11,12 Otherwise, the duration, character, and location of pain were similar to those observed in our cases. Four of the five cases provided detailed neurological findings, and each of these four exhibited ipsilateral facial sensory disturbance (4/4, 100%).6,10-12 Among the three patients with available angiographic data, VA stenosis, dissection, and occlusion were each observed in one patient.6,7,11
We analyzed the time of headache onset after the index stroke and the persistence of the headache in our four cases and five previously reported cases (Table 3). Among these nine patients, five experienced persistent symptoms, and four showed resolution. In the persistent group, headache onset occurred at a mean of 3.24 months (range, 0.7–6 months) after the stroke. In contrast, the resolved group showed a mean onset time of 0.76 months (range, 0–2.5 months).
DISCUSSION
Secondary TACs are relatively uncommon and are related to a wide range of structural brain lesions, including neoplasms, vascular malformations, demyelinating diseases, arterial dissections, and infarctions.3,6,7,9,11,13,14 Among these, dorsolateral medullary infarction—also known as Wallenberg syndrome—is a rare but increasingly recognized etiology of secondary TACs, particularly those resembling SUNCT and SUNA phenotypes.6,7,10-13
In our case series, all four patients developed headache syndromes, consistent with secondary TACs, 2.5 to 4 months after unilateral dorsolateral medullary infarction. The pain was characterized by brief, stabbing or electric shock-like episodes in the ipsilateral periorbital and hemifacial regions, accompanied by prominent cranial autonomic symptoms such as lacrimation and conjunctival injection. The clinical features in three patients met the ICHD-3 diagnostic criteria for SUNCT, while one patient met the criteria for SUNA. This predominance of SUNCT/SUNA, rather than cluster headache, is consistent with prior reports of brainstem-related secondary TACs.3,6,7,9,11,13 Conversely, differentiating between TN-like features and SUNCT/SUNA is often clinically challenging because of overlapping clinical features, as illustrated by a case in our literature review by Lambru et al.,7 which demonstrated coexisting SUNCT- and TN-like features due to dorsolateral medullary hemorrhagic infarction.4,15,16 In Lambru et al.,7 the patient with dorsolateral medullary infarction expected two distinct types of attacks: one with short-lasting stabbing pain (5–20 seconds) accompanied by cranial autonomic symptoms such as lacrimation and conjunctival injection, fulfilling the diagnostic criteria of SUNCT; another type, similarly localized, but triggered by innocuous stimuli (e.g., touch, wind), lacking autonomic signs, and featuring a dull post-attack ache, consistent with TN. The coexistence of these patterns led the authors to diagnose concurrent SUNCT and TN, rather than a single disorder.7 Traditionally, TN is characterized by the involvement of second or third divisions in the trigeminal nerve, a refractory period following exposure to triggers, and the absence of cranial autonomic symptoms.4,7,15,16 In contrast, SUNCT/SUNA typically involves the first division of the trigeminal nerve, presents with longer-lasting attacks (up to 600 seconds), lacks a refractory period, and prominently features autonomic symptoms.4,7,15,16 However, several cases of TN may occasionally present with mild cranial autonomic features, meaning the presence or absence of a refractory period can be difficult to assess, especially in patients with complex stroke-related presentations.7,15,16 In our current series, all patients reported cranial autonomic symptoms, and none had a definite refractory period. Moreover, none of the attacks were clearly stimulus-provoked in a way consistent with TN. Based on these clinical characteristics, these patients were diagnosed with SUNCT/SUNA rather than TN.
While some post-stroke pain syndromes may emerge in the acute phase, others, including central post-stroke pain, can have a delayed onset.17 Similarly, secondary SUNCT/SUNA in our series tended to develop in the subacute or even chronic phase following the index infarction. Notably, our findings suggest that the timing of headache onset may have prognostic implications. Among the nine patients analyzed (our four cases and five from the literature), those with persistent headache symptoms had a later onset (mean, 3.24 months) compared to those whose symptoms eventually resolved (mean, 0.76 months). This observation raises the possibility that delayed onset is associated with a greater risk of chronification, potentially reflecting distinct pathophysiological mechanisms, such as central sensitization or maladaptive reorganization, within trigeminal–autonomic pathways.18,19 These findings underlie the importance of temporal profiling in secondary TACs and emphasize the need for prospective studies with larger cohorts to clarify the temporal relationship between infarction and headache onset in secondary TACs.
Although the clinical presentation of a cluster headache and SUNCT/SUNA can be quite similar, the headache duration, clustering pattern, and treatment responses (e.g., oxygen therapy or indomethacin) can be used to differentiate these conditions.2,4,20 While the precise mechanisms remain unclear, the characteristic clustering of pain in cluster headache suggests that, in addition to involvement of the trigeminal spinal tract and nucleus at the medullary level, dysfunctions of the hypothalamus and the broader trigeminal–autonomic network may also play a significant role.21-23 Consequently, we speculate that the more complex pathophysiology of cluster headache may explain the lower observed incidence of this condition compared to SUNCT/SUNA in cases of isolated dorsolateral medullary infarctions. Nonetheless, further research is warranted to prove our hypothesis.
A cerebral infarction involving the dorsolateral medulla, commonly referred to as Wallenberg syndrome, presents with a constellation of neurological signs, including ipsilateral facial sensory disturbances, contralateral hemibody sensory loss, ataxia, vertigo, and Horner’s syndrome, depending on the specific anatomical structures involved.24 In our series, all four patients exhibited ipsilateral facial sensory deficits (Table 2). Among the five previously published cases of TACs associated with dorsolateral medullary infarction, four provided detailed neurological findings, and all of these described ipsilateral facial sensory loss (Table 2). This consistent pattern across both our cases and the literature strongly implicates the spinal trigeminal tract and nucleus as the key anatomical structures involved in the development of secondary TACs. Although the precise pathomechanisms underlying secondary TACs in dorsolateral medullary infarction remain to be elucidated, it is thought that disruption of the spinal trigeminal tract and nucleus within the dorsolateral medulla may lead to disinhibition or aberrant activation of the trigeminal–autonomic reflex arc.6,18 This may represent the underlying issue attached to the SUNCT or SUNA phenotypes observed in these patients. Given the convergence of nociceptive and autonomic pathways in this region, the spinal trigeminal complex appears to serve as a critical neuroanatomical substrate for secondary TACs following medullary infarction.6,7,13
Importantly, all of our patients exhibited additional focal neurological signs, including crossed sensory loss, nystagmus, ataxia, and Horner’s syndrome, at the time of the index stroke. These neurological features preceded or accompanied the onset of a headache, providing critical diagnostic clues to a secondary etiology. This stands in contrast to other common secondary TACs such as those caused by pituitary tumors or vascular malformations, where diagnosis is frequently delayed for several months to years, mostly due to isolated headache symptoms without overt focal neurological signs.2,25
This study has several limitations. First, this study was not a prospectively designed trial with a predefined, systematic treatment approach for secondary TACs, particularly SUNCT and SUNA. Instead, treatment strategies were heterogeneous across cases, largely reflecting stroke-centered management approaches. Furthermore, patients were initially classified under the broader category of TACs rather than being specifically diagnosed with SUNCT or SUNA. Although the current literature suggests using lamotrigine as a first-line preventive treatment for SUNCT/SUNA,4 none of our four patients received this therapy. Notably, in a previously reported case,10 lamotrigine was associated with substantial symptom resolution. This observation suggests that earlier recognition and targeted preventive therapy may influence long-term outcomes in similar cases.
In conclusion, dorsolateral medullary infarction can be a causative lesion for secondary TACs. Based on our results, most patients exhibited a SUNCT or SUNA phenotype and consistently presented with ipsilateral hemifacial sensory loss. Moreover, the delayed onset of headache was often associated with a reduced response to treatment and persistent headache. Thus, future studies should further investigate the pathophysiological mechanisms and prognostic implications of delayed-onset secondary TACs in brainstem infarctions.
Notes
AVAILABILITY OF DATA AND MATERIAL
The data presented in this study are available upon reasonable request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization: JMK, SHL; Data curation: JMK, HLL, YRK, JTK; Formal analysis: SHL; Investigation: JMK, HLL, YRK, JTK; Validation: SHL; Writing–original draft: JMK, SHL; Writing–review & editing: JMK, HLL, YRK, JTK, SHL.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING STATEMENT
Not applicable.
ACKNOWLEDGMENTS
Not applicable.