Abstract
- Migraine is a prevalent and disabling neurological disorder in which established pathophysiological mechanisms, including trigeminovascular activation and calcitonin gene-related peptide (CGRP) signaling, do not fully account for interindividual susceptibility, chronification, or treatment refractoriness. Advances in neurobiology have drawn attention to brain clearance pathways, specifically the glymphatic system and meningeal lymphatic vessels, as potential modulators of neuroinflammation and cerebrospinal fluid (CSF) dynamics. These systems regulate the exchange and drainage of CSF, interstitial solutes, and immune mediators and are strongly influenced by sleep and state-dependent physiology, both of which are closely linked to migraine pathophysiology. In this narrative review, we describe the anatomical and functional organization of brain lymphatic and glymphatic systems and critically evaluate emerging evidence connecting these pathways to migraine. Indirect human imaging studies and experimental models indicate that alterations in perivascular transport, meningeal lymphatic drainage, sleep disruption, and CGRP-related signaling may converge to modulate brain clearance efficiency in migraine. Although the available evidence remains heterogeneous and largely indirect, these findings offer a coherent framework for integrating clearance-related physiology into existing migraine models. We further discuss neuromodulation as a potential strategy for influencing brain clearance mechanisms. In particular, transcranial low-intensity ultrasound has been shown to enhance CSF movement in vivo, providing direct mechanistic support for clearance modulation. Other neuromodulation modalities may exert indirect effects through autonomic regulation, neural oscillations, or vascular dynamics. While clinical evidence remains preliminary, a clearance-oriented perspective may help guide future biomarker development and translational research in migraine.
-
Keywords: Glymphatic system, Lymphatic system, Migraine disorders, Ultrasonic therapy
INTRODUCTION
Migraine is a common and disabling neurological disorder characterized by recurrent head pain and sensory hypersensitivity. Although therapeutic advances—particularly agents targeting calcitonin gene-related peptide (CGRP)—have markedly improved outcomes for many patients, a substantial proportion continues to experience frequent attacks or progression to chronic migraine. This observation underscores the need for complementary mechanistic frameworks that can provide additional insights into disease susceptibility and therapeutic targets. Beyond ictal headache episodes, migraine is increasingly recognized as a disorder associated with a substantial interictal burden, including persistent sensory and sleep-related disturbances. These features suggest that migraine-related pathophysiology may extend beyond discrete attacks and involve ongoing alterations in brain physiology between episodes, providing a relevant context for considering brain clearance mechanisms.1
Recent advances in neurobiology have identified specialized brain clearance pathways that contribute to the removal of interstitial solutes and inflammatory mediators. The glymphatic system facilitates cerebrospinal fluid (CSF)-interstitial fluid (ISF) exchange along perivascular spaces and appears to play a central role in metabolic waste clearance and immune signaling in the brain. Dysfunction of the glymphatic pathway has been implicated in a variety of neurological conditions and is proposed to contribute to headache pathophysiology as well.2,3 In migraine, indirect imaging markers such as altered diffusion metrics along perivascular spaces have been interpreted as suggesting impaired glymphatic transport, consistent with emerging evidence of glymphatic disturbance in this disorder.4
Complementing glymphatic exchange, meningeal lymphatic vessels provide an anatomically distinct outflow route for CSF-derived solutes and immune cells toward deep cervical lymph nodes, linking central nervous system fluid dynamics with peripheral immune surveillance.5
Importantly, glymphatic function is strongly influenced by sleep and state-dependent physiology. Glymphatic transport is enhanced during sleep-like conditions, which may be relevant to migraine given the well-established interactions between sleep disruption and headache disorders.6 Emerging imaging studies in humans have begun to explore clearance-related signatures in migraine, including meningeal lymphatic drainage abnormalities and alterations in perivascular fluid dynamics, although these findings are heterogeneous and require further validation.7,8
Mechanistically, CGRP signaling—a key driver of migraine pain—not only contributes to neurovascular inflammation but may also influence meningeal lymphatic function, thereby providing a possible link between classical migraine biology and brain clearance pathways.3 From a therapeutic standpoint, the brain clearance framework suggests novel interventional opportunities. Neuromodulation may influence CSF movement and glymphatic exchange in ways that extend beyond neural excitability modulation.
In vivo imaging evidence has shown that transcranial focused ultrasound can enhance CSF movement and facilitate waste clearance in experimental models, providing a potential mechanistic basis for its exploration in migraine.9 In addition, emerging translational studies, including our recent in-press work 10 support the broader concept that low-intensity ultrasound may modulate glymphatic-related processes in humans.
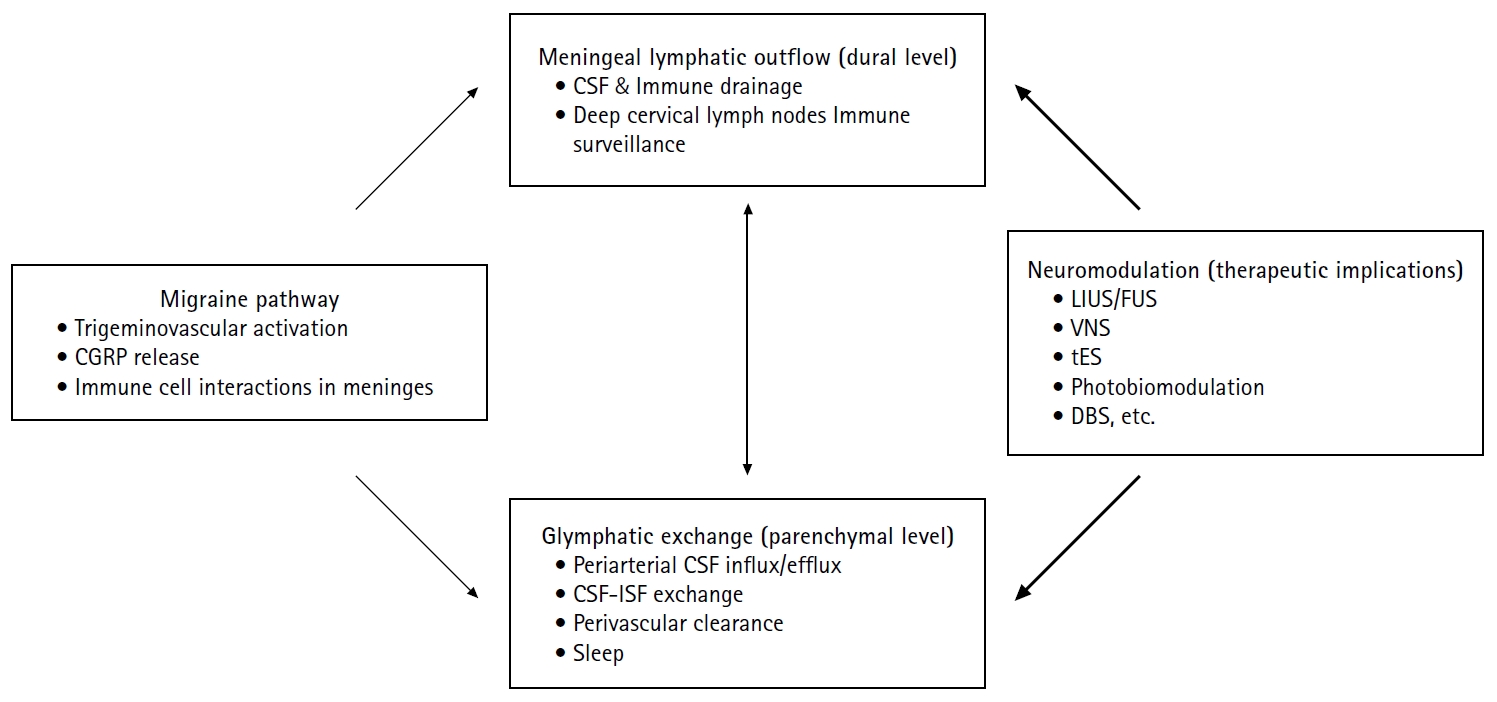
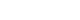
In this review, we provide a concise overview of brain glymphatic and lymphatic systems, summarize evidence linking these pathways to migraine, and discuss the therapeutic implications of neuromodulation—with a focus on ultrasound-based approaches. By integrating mechanistic insight with clinical perspective, we aim to present a pragmatic framework for future biomarker development and translational research in migraine. Figure 1 summarizes the proposed interactions between migraine pathophysiology, brain clearance systems, and neuromodulation discussed in this review.
OVERVIEW OF BRAIN CLEARANCE SYSTEMS
Brain homeostasis depends on specialized clearance pathways that regulate the movement and removal of CSF, interstitial solutes, and immune signals. Two anatomically and functionally distinct but interconnected systems are central to this process: the glymphatic system and the meningeal lymphatic system.
1. Glymphatic system
The glymphatic system operates at the parenchymal level and facilitates convective exchange between CSF and ISF along perivascular spaces. CSF enters the brain predominantly via periarterial pathways, mixes with ISF within the parenchyma, and exits along perivenous routes, thereby contributing to the clearance of metabolic byproducts and neuroactive solutes.11 Glymphatic transport is strongly state dependent, with enhanced CSF-ISF exchange during sleep and suppression during wakefulness, reflecting physiological regulation of brain waste clearance.12 From a clinical standpoint, this framework is relevant to migraine, a disorder in which sleep disturbance is both a common trigger and a frequent consequence of attacks, potentially predisposing patients to impaired brain clearance.
2. Meningeal lymphatic system
In contrast to the parenchymal glymphatic pathway, the meningeal lymphatic system functions at the dural level and represents a conventional lymphatic outflow route. Meningeal lymphatic vessels drain CSF-derived solutes, macromolecules, and immune cells toward the deep cervical lymph nodes, linking intracranial fluid dynamics with peripheral immune surveillance.13,14 Because migraine pain arises from meningeal and trigeminovascular structures, alterations in meningeal lymphatic drainage may be particularly relevant to migraine pathophysiology.
3. Functional interaction and clinical relevance
Although distinct, the glymphatic and meningeal lymphatic systems are functionally interconnected. Solutes cleared from the brain parenchyma via glymphatic pathways ultimately rely on meningeal lymphatic vessels for extracranial drainage, and dysfunction at either level may compromise overall brain clearance efficiency.13-15 In migraine, mechanisms such as sleep disturbance and neurogenic inflammation may disrupt this coordinated clearance network, providing a conceptual basis for emerging biomarker and therapeutic research.
EVIDENCE OF LYMPHATIC AND GLYMPHATIC DYSFUNCTION IN MIGRAINE
Current evidence linking migraine to dysfunction of brain clearance systems remains emerging and heterogeneous. Rather than definitive proof, the available data collectively suggest that alterations in CSF dynamics, perivascular transport, and meningeal immune drainage may accompany migraine susceptibility and chronification.
1. Indirect human imaging evidence of altered brain clearance
In humans, direct visualization of glymphatic flow remains technically challenging. As a result, most clinical evidence in migraine relies on indirect imaging markers that reflect perivascular fluid dynamics. Diffusion tensor imaging–based analysis along perivascular spaces (DTI-ALPS) has been proposed as a surrogate marker of glymphatic function and has been applied to patients with migraine. DTI-ALPS is based on the anatomical observation that perivascular spaces surrounding penetrating arteries and veins serve as major conduits for glymphatic fluid transport. Because diffusion along these spaces is directionally constrained, DTI can be used to estimate preferential water diffusivity parallel to perivascular pathways. The ALPS index therefore provides an indirect surrogate of perivascular fluid movement rather than a direct measure of glymphatic flow. Several studies report altered ALPS indices or perivascular imaging features in migraine compared with controls, although the direction and magnitude of these changes vary across cohorts.3
In migraine, DTI-ALPS studies have reported heterogeneous findings. Some studies suggest altered ALPS indices in chronic migraine compared with episodic migraine or controls, whereas data comparing migraine with and without aura remain limited and inconsistent. Importantly, several studies have reported null or variable results, and correlations between ALPS indices and clinical variables such as headache frequency or disease duration have not been consistently demonstrated.3,4,8 Additional radiological findings associated with migraine, such as enlarged perivascular spaces and white matter hyperintensities-have also been discussed in the context of impaired ISF clearance.16 However, these features are nonspecific and may reflect cumulative vascular, inflammatory, or aging-related processes rather than direct evidence of glymphatic dysfunction.
2. Experimental evidence linking migraine-related mechanisms to meningeal lymphatics
Beyond imaging, experimental studies provide more direct mechanistic insight. Recent preclinical work has demonstrated that meningeal lymphatic vessels are functionally responsive to migraine-related signaling pathways. In particular, CGRP signaling—central to migraine pathophysiology—has been shown to modulate meningeal lymphatic function, influencing CSF efflux, immune interactions, and pain-related behaviors in animal models. These findings suggest that meningeal lymphatics may represent an interface through which trigeminovascular activation and immune signaling converge.17
3. Sleep disturbance as a modifier of clearance in migraine
Sleep disturbance is one of the most consistent clinical features associated with migraine, acting as both a trigger and a consequence of attacks. Experimental studies outside the migraine field demonstrate that sleep strongly enhances glymphatic transport, whereas sleep deprivation suppresses CSF-ISF exchange. In migraine, recurrent sleep disruption may therefore represent a physiological mechanism by which brain clearance efficiency is reduced, potentially facilitating the accumulation of pro-inflammatory or pro-nociceptive mediators.12,18
4. Limitations of current evidence
Despite growing interest, several limitations should be acknowledged. Human studies largely rely on indirect imaging markers with variable reproducibility, while experimental models may not fully capture the complexity of migraine phenotypes. Moreover, whether observed clearance alterations are a cause, consequence, or epiphenomenon of migraine remains unresolved. These gaps underscore the need for standardized biomarkers and longitudinal studies integrating imaging, physiological measures, and clinical outcomes.
NEUROMODULATION AS A STRATEGY TO MODULATE BRAIN CLEARANCE
Neuromodulation refers to the application of electrical, magnetic, or acoustic stimulation to alter neural activity or physiological processes, and has been increasingly explored in migraine for both preventive and acute treatment. Traditionally, neuromodulation strategies have focused on modulating cortical excitability or nociceptive processing. In the context of brain clearance, however, neuromodulation may additionally influence CSF dynamics, vascular pulsatility, and state-dependent physiology. The brain clearance framework adds an additional, clinically testable dimension: whether neuromodulation can influence CSF dynamics and, by extension, glymphatic–lymphatic function. This perspective is particularly relevant because clearance pathways are state dependent and are tightly coupled to sleep physiology, arousal, and neurovascular tone.
1. Why neuromodulation is conceptually attractive in a clearance framework
From a translational standpoint, clearance dysfunction—if present in migraine—may not be optimally addressed by conventional analgesic or anti-inflammatory approaches alone. Neuromodulation offers a potentially complementary route by targeting upstream physiology that shapes CSF movement (e.g., vascular pulsatility, perivascular transport, and state-dependent fluid exchange). In this model, the therapeutic hypothesis is not limited to “pain inhibition,” but extends to “restoring a physiological state that facilitates clearance.”
2. Transcranial ultrasound: direct in vivo evidence of enhanced cerebrospinal fluid movement and clearance modulation
Among neuromodulation modalities, transcranial low-intensity ultrasound has particular relevance because it has been shown using real-time optical imaging to enhance CSF movement and cortical CSF influx in vivo, providing mechanistic support for the concept that stimulation may influence clearance-related fluid dynamics.9 Clinically, this evidence is valuable because it establishes a measurable intermediate endpoint (CSF movement/CSF influx) that can be linked to imaging biomarkers and, ultimately, headache outcomes in future trials.
Consistent with this physiological effect on CSF dynamics, a related body of work in neurodegeneration provides proof-of-concept that ultrasound-based interventions can influence glymphatic–lymphatic drainage at a systems level. Focused ultrasound combined with microbubbles has been reported to enhance the drainage of soluble amyloid-β from the brain to CSF spaces and onward toward deep cervical lymph nodes, supporting the broader plausibility of ultrasound-mediated clearance modulation.19 This line of evidence does not imply direct clinical efficacy for migraine, but it strengthens the mechanistic rationale that clearance pathways are modifiable rather than purely passive.
3. Other neuromodulation modalities
Beyond transcranial ultrasound, other neuromodulation techniques have been investigated for their potential influence on CSF dynamics and brain clearance pathways. Clinically patterned vagus nerve stimulation (VNS) has been shown to enhance CSF tracer penetrance in animal models, suggesting that VNS may alter CSF/ISF exchange and clearance mechanisms.20 Low-frequency auricular VNS increased arterial vasomotion and cortical CSF influx in vivo, further supporting the concept that modulation of autonomic pathways can influence perivascular fluid movement.21 Transcranial electrical stimulation (tES) modalities, such as transcranial direct current or alternating current stimulation, primarily target cortical excitability but also influence neural oscillations and neurovascular coupling. While direct evidence linking tES to glymphatic clearance is currently limited, recent studies demonstrate that slow vascular and neural rhythms—which can be influenced by neuromodulatory interventions such as tES-are associated with enhanced CSF movement and glymphatic function during states such as non-rapid eye movement sleep.22,23 Photobiomodulation has been proposed to augment brain lymphatic and glymphatic drainage in preclinical models, potentially via vasodilatory effects on vascular and lymphatic pathways.24 Although these modalities do not yet have direct clinical evidence of clearance modulation in migraine, their physiological effects on autonomic tone, vascular dynamics, or neural rhythms provide a plausible mechanistic basis for future investigation in headache and pain disorders.
In addition to clearance-oriented neuromodulation strategies, several neuromodulation approaches have been investigated in migraine and pain disorders, primarily with the aim of modulating nociceptive processing or cortical excitability. For example, noninvasive stimulation techniques, including motor cortex stimulation and other brain stimulation paradigms, have demonstrated modulatory effects on pain perception and migraine-related symptoms in both experimental and clinical settings.25-27 These approaches are generally conceptualized as preventive or abortive interventions depending on stimulation timing and target engagement. In contrast, focused ultrasound-based neuromodulation may occupy a distinct conceptual position. Rather than directly targeting pain networks, ultrasound has been shown to influence CSF movement and perivascular transport, suggesting a potential role in modulating the physiological state that shapes migraine susceptibility. From this perspective, ultrasound-based approaches may be more appropriately framed as modulators of underlying brain physiology, which could complement established preventive or abortive strategies rather than replace them.
4. Translational implications for migraine
In migraine, a clearance-oriented neuromodulation strategy is best framed as hypothesis-driven and staged. Near-term goals include identifying patients with the most convincing clearance-related signatures (e.g., indirect glymphatic markers or meningeal lymphatic-related imaging patterns), and testing whether neuromodulation produces measurable changes in CSF dynamics and clearance surrogates alongside clinical endpoints such as attack frequency and disability. Longer-term, mechanistic integration with established migraine biology-particularly meningeal neuroinflammation and CGRP-linked pathways-may help determine whether “clearance modulation” represents a distinct therapeutic mechanism or a convergent downstream effect.
CLINICAL PERSPECTIVES AND FUTURE DIRECTIONS
Emerging evidence suggests that brain lymphatic and glymphatic systems may contribute to migraine biology, but their clinical relevance remains to be clearly defined. Rather than proposing clearance modulation as a stand-alone therapeutic paradigm, future research should focus on how clearance-related mechanisms can refine biomarker development, patient stratification, and translational study design.
1. Biomarkers and patient stratification
At present, assessment of brain clearance function in humans relies largely on indirect imaging markers, including perivascular diffusion-based metrics and radiological features associated with altered ISF dynamics. While these measures are imperfect, they provide a practical foundation for exploratory studies in migraine, particularly in patients with chronic migraine, prominent sleep disturbance, or treatment-refractory disease. Standardization and validation across centers will be essential before such markers can inform routine clinical decision-making.3,12
2. Translational implications for neuromodulation
Neuromodulation approaches may offer a means to influence physiological processes that shape CSF dynamics and clearance efficiency. Among these, focused ultrasound is of particular interest because it has been shown to enhance CSF movement in vivo, providing a measurable intermediate endpoint that can be integrated into translational study designs. Importantly, early-phase studies should combine clinical outcomes with physiological or imaging-based measures to clarify whether neuromodulation effects are mediated through clearance-related mechanisms, pain network modulation, or both.9,19
3. Integration with established migraine mechanisms
Clearance-based concepts should be viewed as complementary to established migraine biology rather than as competing models. Interactions among CGRP signaling, meningeal immune activation, sleep disruption, and clearance efficiency may converge to influence migraine susceptibility and long-term disease course. Determining whether altered clearance represents a driver, amplifier, or downstream consequence of migraine attacks will require carefully designed longitudinal and interventional studies.13,17
CONCLUSION
Emerging evidence suggests that brain lymphatic and glymphatic systems represent a relevant but still evolving dimension of migraine biology. While current data do not establish clearance dysfunction as a primary driver of migraine, they provide a coherent framework for integrating sleep physiology, meningeal immune interactions, and CSF dynamics into existing pathophysiological models. Continued translational research combining mechanistic insight with clinically grounded study design will determine whether modulation of brain clearance pathways can meaningfully contribute to future migraine management.
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
AUTHOR CONTRIBUTIONS
Conceptualization: JK; Writing–original draft: JK; Writing–review & editing: JK.
CONFLICT OF INTEREST
Author has served as an advisory consultant for DeepsonBio. This role was not related to the content of the present review and the author has no other conflicts of interest to declare.
FUNDING STATEMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2022-NR072047), and supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (No. GTL25071-000).
ACKNOWLEDGMENTS
Not applicable.
Figure 1.
Proposed interactions between migraine pathophysiology, brain clearance systems, and neuromodulation. Migraine involves trigeminovascular activation with CGRP release and neuroimmune interactions in the meninges. Brain clearance operates at two interconnected levels: glymphatic exchange within the parenchyma, characterized by periarterial CSF influx, CSF-ISF exchange, and perivascular clearance that are modulated by sleep; and meningeal lymphatic outflow at the dural level, which drains CSF-derived solutes and immune components toward deep cervical lymph nodes. Experimental evidence suggests that migraine-related mechanisms, including CGRP signaling and sleep disruption, may impair these clearance pathways. Neuromodulation strategies—such as low-intensity or FUS and other noninvasive modalities—may influence CSF dynamics and glymphatic–lymphatic function, representing a potential therapeutic approach for modulating migraine susceptibility. Solid arrows indicate experimentally supported relationships, whereas bold arrows denote hypothesized interactions.
CGRP, calcitonin gene-related peptide; CSF, cerebrospinal fluid; ISF, interstitial fluid; LIUS, low-intensity ultrasound stimulation; FUS, focused ultrasound; VNS, vagus nerve stimulation; tES, transcranial electrical stimulation; DBS, deep brain stimulation.
REFERENCES
- 1. Kim SK, Schwedt TJ. Interictal burden of migraine: a narrative review. Headache Pain Res 2025;26:200-208.ArticlePDF
- 2. Yankova G, Bogomyakova O, Tulupov A. The glymphatic system and meningeal lymphatics of the brain: new understanding of brain clearance. Rev Neurosci 2021;32:693-705.ArticlePubMed
- 3. Vittorini MG, Sahin A, Trojan A, et al. The glymphatic system in migraine and other headaches. J Headache Pain 2024;25:34.ArticlePubMedPMCPDF
- 4. Cha MJ, Kang KW, Shin JW, Kim H, Kim J. Understanding the connection between the glymphatic system and migraine: a systematic review. Headache Pain Res 2024;25:86-95.ArticlePDF
- 5. Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019;572:62-66.ArticlePubMedPDF
- 6. Hablitz LM, Plá V, Giannetto M, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun 2020;11:4411.ArticlePubMedPMCPDF
- 7. Ren X, Wang H, He Y, et al. Impaired meningeal lymphatic drainage correlates with headache intensity in episodic migraine. J Headache Pain 2025;26:265.ArticlePubMedPMCPDF
- 8. Zhang X, Wang W, Bai X, et al. Increased glymphatic system activity in migraine chronification by diffusion tensor image analysis along the perivascular space. J Headache Pain 2023;24:147.ArticlePubMedPMCPDF
- 9. Choi S, Kum J, Hyun SY, et al. Transcranial focused ultrasound stimulation enhances cerebrospinal fluid movement: real-time in vivo two-photon and widefield imaging evidence. Brain Stimul 2024;17:1119-1130.ArticlePubMed
- 10. Jo SW, Park B, Yoo R, et al. Effects of low-intensity ultrasound on amyloid-β clearance in early-stage Alzheimer’s disease: a potential role of glymphatic activity. Acta Neurol Scand. Forthcoming 2026. https://doi.org/10.1155/ane/1538313.Article
- 11. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111.ArticlePubMedPMC
- 12. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373-377.ArticlePubMedPMC
- 13. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015;523:337-341.ArticlePubMedPMCPDF
- 14. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015;212:991-999.ArticlePubMedPMCPDF
- 15. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018;17:1016-1024.Article
- 16. Cho JW, Lee GH, Kim J. Chronic migraine is associated with region-specific high-grade enlarged perivascular spaces: retrospective matched case-control study. J Clin Neurol 2026;22:113-121.ArticlePubMedPMCPDF
- 17. Nelson-Maney NP, Bálint L, Beeson AL, et al. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J Clin Invest 2024;134:e175616.ArticlePubMedPMC
- 18. He Z, Sun J. Clearance mechanisms of the glymphatic/lymphatic system in the brain: new therapeutic perspectives for cognitive impairment. Cogn Neurodyn 2025;19:111.ArticlePubMedPMCPDF
- 19. Lee Y, Choi Y, Park EJ, et al. Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer’s disease model. Sci Rep 2020;10:16144.ArticlePubMedPMCPDF
- 20. Cheng KP, Brodnick SK, Blanz SL, et al. Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul 2020;13:1024-1030.ArticlePubMed
- 21. Choi S, Baek IS, Lee K, Kim SK. Low-frequency auricular vagus nerve stimulation facilitates cerebrospinal fluid influx by promoting vasomotion. Korean J Physiol Pharmacol 2025;29:109-116.ArticlePubMedPMC
- 22. Hauglund NL, Andersen M, Tokarska K, et al. Norepinephrine-mediated slow vasomotion drives glymphatic clearance during sleep. Cell 2025;188:606-622.e17.ArticlePubMedPMC
- 23. Wang Y, Monai H. Transcranial direct current stimulation alters cerebrospinal fluid-interstitial fluid exchange in mouse brain. Brain Stimul 2024;17:620-632.ArticlePubMed
- 24. Salehpour F, Khademi M, Bragin DE, DiDuro JO. Photobiomodulation therapy and the glymphatic system: promising applications for augmenting the brain lymphatic drainage system. Int J Mol Sci 2022;23:2975.ArticlePubMedPMC
- 25. Yao L, Chen R, Ji H, Wang X, Zhang X, Yuan Y. Preventive and therapeutic effects of low-intensity ultrasound stimulation on migraine in rats. IEEE Trans Neural Syst Rehabil Eng 2022;30:2332-2340.ArticlePubMed
- 26. Badran BW, Caulfield KA, Stomberg-Firestein S, et al. Sonication of the anterior thalamus with MRI-Guided transcranial focused ultrasound (tFUS) alters pain thresholds in healthy adults: a double-blind, sham-controlled study. Brain Stimul 2020;13:1805-1812.ArticlePubMedPMC
- 27. Riis TS, Feldman DA, Losser AJ, Okifuji A, Kubanek J. Noninvasive targeted modulation of pain circuits with focused ultrasonic waves. Pain 2024;165:2829-2839.ArticlePubMedPMC
Citations
Citations to this article as recorded by




 PubReader
PubReader ePub Link
ePub Link Cite this Article
Cite this Article
 KHS
KHS