Articles
- Page Path
- HOME > Headache Pain Res > Volume 25(1); 2024 > Article
-
Review Article
Update on Cluster Headaches: From Genetic to Novel Therapeutic Approaches -
Myun Kim*
 , Je Kook Yu*
, Je Kook Yu* , Yoo Hwan Kim
, Yoo Hwan Kim
-
Headache and Pain Research 2024;25(1):42-53.
DOI: https://doi.org/10.62087/hpr.2024.0009
Published online: April 22, 2024
Department of Neurology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Republic of Korea
- Corresponding author: Yoo Hwan Kim, M.D., Ph.D. Department of Neurology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, 22 Gwanpyeong-ro 170beon-gil, Dongan-gu, Anyang 14068, Republic of Korea Tel: +82-31-380-3740, Fax: +82-31-380-4118, E-mail: drneuroneo@gmail.com
- *These authors contributed equally to this study as co-first authors.
• Received: February 17, 2024 • Revised: March 8, 2024 • Accepted: March 11, 2024
© 2024 The Korean Headache Society
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 17,602 Views
- 161 Download
- 10 Crossref
Figure & Data
References
Citations
Citations to this article as recorded by 

- Exercise as an abortive treatment for cluster headaches: Insights from a large patient registry
Mi‐Kyoung Kang, Yooha Hong, Soo‐Jin Cho
Annals of Clinical and Translational Neurology.2025; 12(1): 149. CrossRef - Morning Headaches: An In-depth Review of Causes, Associated Disorders, and Management Strategies
Yooha Hong, Mi-Kyoung Kang, Min Seung Kim, Heejung Mo, Rebecca C. Cox, Hee-Jin Im
Headache and Pain Research.2025; 26(1): 66. CrossRef - Does Laterality Matter? Insights Into Unilateral Pain in Cluster Headache
Tae-Jin Song
Journal of Clinical Neurology.2025; 21(3): 157. CrossRef - Pain Lateralization in Cluster Headache and Associated Clinical Factors
Soohyun Cho, Mi Ji Lee, Min Kyung Chu, Jeong Wook Park, Heui-Soo Moon, Pil-Wook Chung, Jong-Hee Sohn, Byung-Su Kim, Daeyoung Kim, Kyungmi Oh, Byung-Kun Kim, Soo-Jin Cho
Journal of Clinical Neurology.2025; 21(3): 220. CrossRef - Subtype shift, relapse rate and risk factors of frequent relapse in cluster headache: A multicenter, prospective, longitudinal observation
Mi Ji Lee, Soo-Kyoung Kim, Min Kyung Chu, Jae Myun Chung, Heui-Soo Moon, Pil-Wook Chung, Jeong Wook Park, Byung-Kun Kim, Kyungmi Oh, Yun-Ju Choi, Jong-Hee Sohn, Byung-Su Kim, Dae Woong Bae, Daeyoung Kim, Tae-Jin Song, Kwang-Yeol Park, Soo-Jin Cho
Cephalalgia.2025;[Epub] CrossRef - Inverse association of obesity with bout periodicity in episodic cluster headache: a multicenter cross-sectional study
Byung-Su Kim, Mi Ji Lee, Byung-Kun Kim, Jong-Hee Sohn, Tae-Jin Song, Min Kyung Chu, Soo-Kyoung Kim, Jeong Wook Park, Heui-Soo Moon, Pil-Wook Chung, Soo-Jin Cho
The Journal of Headache and Pain.2025;[Epub] CrossRef - Trigeminal Autonomic Cephalalgias Following Unilateral Dorsolateral Medullary Infarction: A Case Series and Literature Review
Jae-Myung Kim, Hak-Loh Lee, You-Ri Kang, Joon-Tae Kim, Seung-Han Lee
Headache and Pain Research.2025; 26(3): 218. CrossRef - Isolated Dental and Lower-Facial Pain Mimicking Trigeminal Neuropathy: An Indirect Carotid-Cavernous Fistula
Byoungchul Choi, Chulho Kim, Sung-Hwan Kim, Jong-Hee Sohn
Headache and Pain Research.2025; 26(3): 226. CrossRef - Side Shift of Attacks in Cluster Headache: A Prospective Single-center Study
Michelle Sojung Youn, Jun Pyo Kim, Mi Ji Lee
Headache and Pain Research.2024; 25(2): 96. CrossRef - Reduction of neck pain severity in patients with medication-overuse headache
Yooha Hong, Hong-Kyun Park, Mi-Kyoung Kang, Sun-Young Oh, Jin-Ju Kang, Heui-Soo Moon, Tae-Jin Song, Mi Ji Lee, Min Kyung Chu, Soo-Jin Cho
The Journal of Headache and Pain.2024;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Update on Cluster Headaches: From Genetic to Novel Therapeutic Approaches
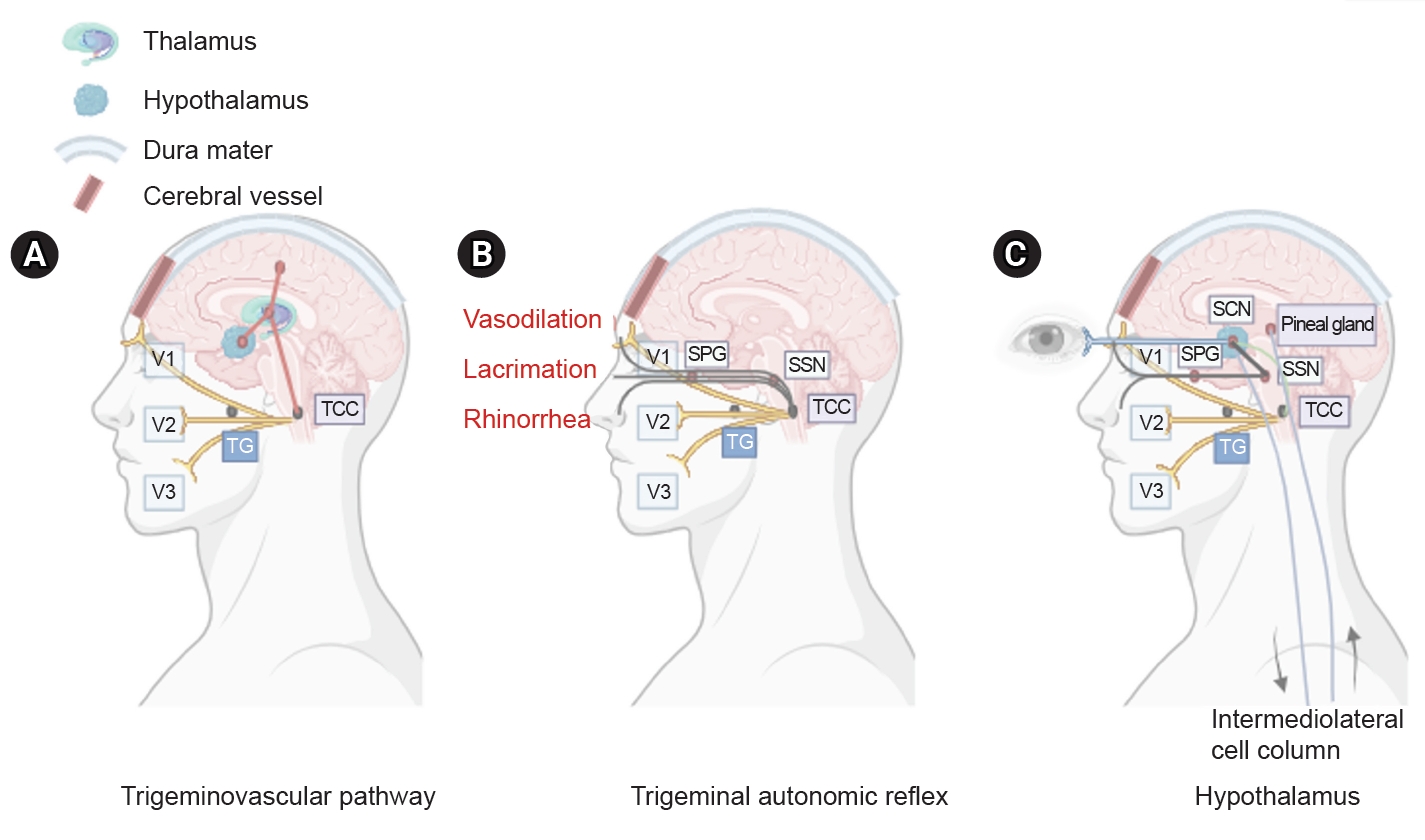
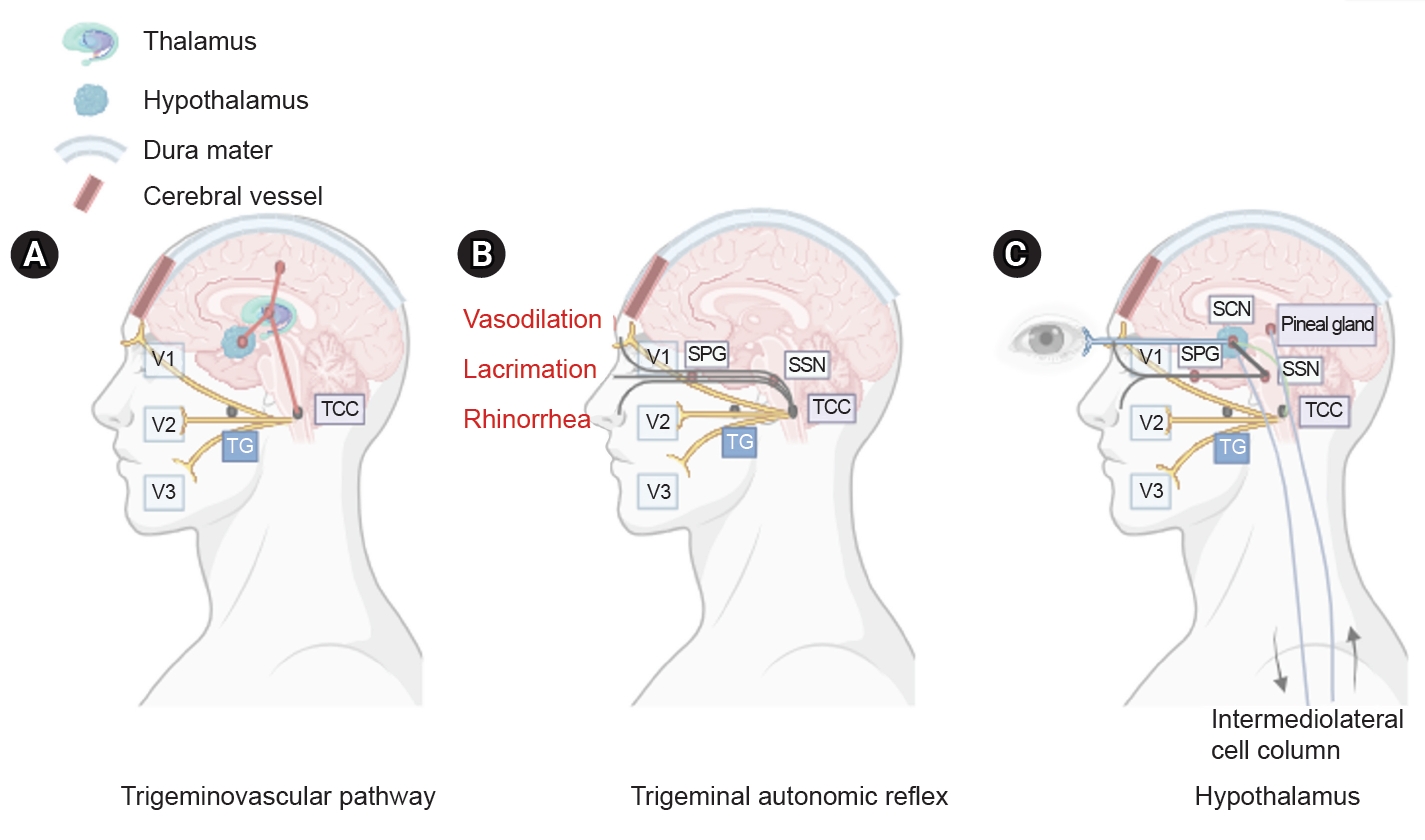
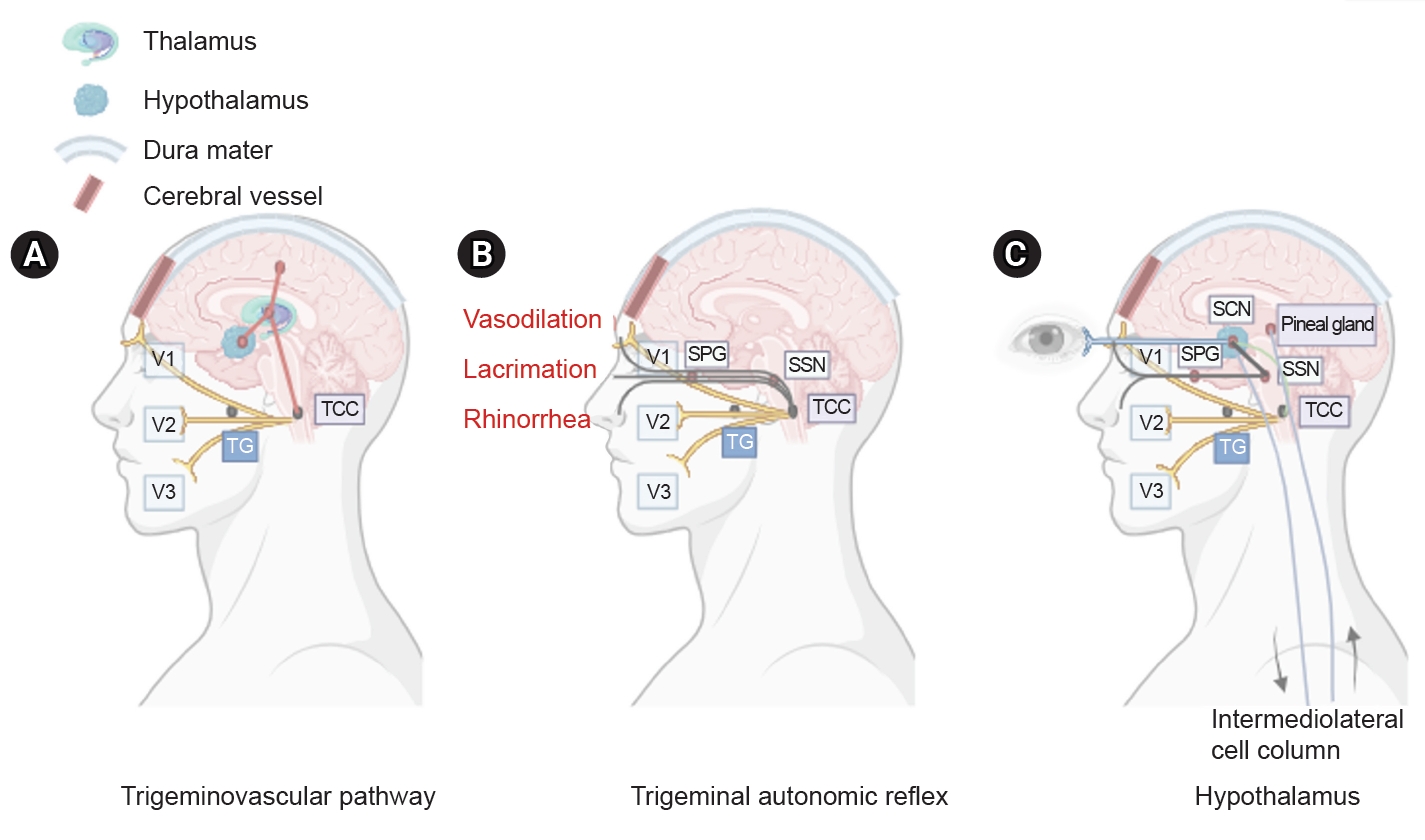
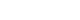
Figure 1. Three main components of cluster headache pathophysiology. (A) The trigeminal ganglion (TG) innervates the cerebral vessels and dura mater through its trigeminal branches (V1, V2, and V3) and forms synapses in the center of the trigeminocervical complex (TCC). Projections of cervical nerves from the TCC to the thalamus activate cortical structures involved in pain processing, such as the prefrontal cortex, subcortex, and cingulate cortex. (B) Trigeminal nerve terminals activate secondary TCC neurons that project to the superior salivary nucleus (SSN) of the pons and from SSN synapses to the peripheral sphenopalatine ganglion (SPG). The postganglionic parasympathetic nerves then innervate the lacrimal, nasal, and pharyngeal glands, causing autonomic symptoms. (C) The suprachiasmatic nucleus (SCN) of the hypothalamus receives light impulse input from the retina via the retinohypothalamus, and these light impulses are transmitted to the paraventricular nucleus and then to the medial and lateral nuclei of the spinal cord, supporting postganglionic sympathetic axons. The hypothalamic region projects directly to the SSN, which in turn projects to the SPG, and nerves project to the lacrimal, nasal, and pharyngeal glands.
Figure 1.
Update on Cluster Headaches: From Genetic to Novel Therapeutic Approaches
| Diagnostic criteria of cluster headache (ICHD-2) | Diagnostic criteria of cluster headache (ICHD-3) |
| A. At least five attacks fulfilling criteria B–D | A. At least five attacks fulfilling criteria B–D |
| B. Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15–180 minutes if untreated | B. Severe or very severe unilateral orbital, supraorbital and/or temporal pain lasting 15–180 minutes (when untreated) |
| C. Headache is accompanied by at least one of the following: | C. Either or both of the following: |
| 1. Ipsilateral conjunctival injection and/or lacrimation | 1. at least one of the following symptoms or signs, ipsilateral to the headache: |
| 2. Ipsilateral nasal congestion and/or rhinorrhea | a) conjunctival injection and/or lacrimation |
| 3. Ipsilateral eyelid edema | b) nasal congestion and/or rhinorrhea |
| 4. Ipsilateral forehead and facial sweating | c) eyelid edema |
| 5. Ipsilateral miosis and/or ptosis | d) forehead and facial sweating |
| 6. A sense of restlessness or agitation | e) miosis and/or ptosis |
| D. Attacks have a frequency from one every other day to 8 per day | 2. a sense of restlessness or agitation |
| E. Not attributed to another disorder | D. Occurring with a frequency between one every other day and eight per day |
| E. Not better accounted for by another ICHD-3 diagnosis. |
| Treatment | Dose | Evidence | Adverse events |
|---|---|---|---|
| Treatment of acute cluster attacks | |||
| Oxygen | 12 L/min, 100% | +++ | - |
| Sumatriptan s.c. | 6 mg | +++ | Feeling of pressure, warmth, heaviness, chest pain, local reaction at the injection site, drowsiness, feeling of weakness, increase or decrease in blood pressure, bradycardia, tachycardia |
| Sumatriptan nasal spray | 20 mg | ++ | |
| Zolmitriptan nasal spray | 5 mg | ++ | |
| Bridging therapy for cluster headaches | |||
| Prednisone | 100 mg tapering by 20 mg every 2–3 days | ++ | Depression, irritability, euphoria, stomach problems, GI ulcer, blood glucose increase, sleep disorders |
| Greater occipital nerve block | ++ | Local irritation | |
| Preventive therapy for cluster headaches | |||
| Verapamil | 200–960 mg | ++ | Hypotension, fatigue, constipation, edema, bradycardia, AV block |
| Lithium | ++ | Tremor, acne, goiter, hypothyroidism, muscle weakness | |
| Topiramate | 100–150 mg | + | Cognitive dysfunction, fatigue, dizziness, paresthesia, mood swings, anxiety, weight loss, hair loss |
| Gabapentin | 1,000–1,800 mg | (+) | Dizziness, somnolence, peripheral edema |
| Melatonin | 10 mg | (+) | Daytime sleepiness, headache dizziness, hypothermia |
| Galcanezumab | 120 mg s.c. once monthly | + | Local reaction, hypersensitivity, constipation |
Table 1. Evolution of the diagnostic criteria for cluster headaches
ICHD, International Classification of Headache Disorder.
Table 2. Treatment of cluster headaches39
s.c., subcutaneous; GI, gastrointestinal; AV, atrioventricular; +++, a high level of evidence from studies; ++, moderate evidence from studies; +, low evidence; (+), questionable evidence.
Table 1.
Table 2.
TOP
 KHS
KHS